Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is becoming a safe and effective technique for treating symptomatic aortic valvular stenosis (AVS) as an alternative to surgery in very high-risk patients. A possible consequence of valve implantation is the obstruction of coronary ostia.
Methods and results: Here we report five cases of angiographically confirmed left main (LM) obstruction, occurred immediately after balloon expandable aortic valve implantations at our institution. In four of these cases, LM obstruction was resolved with an emergency percutaneous coronary intervention (PCI). In the remaining case, obstruction transiently occurred only at the time of balloon valvuloplasty and did not required treatment. During this type of intervention, performing an aortography at the time of balloon valvuloplasty could help to identify patients at risk for coronary obstructions.
Conclusions: These cases illustrate that obstruction of the coronary ostia following TAVI is a possible complication. As the use of TAVI becomes widespread, the operators should be aware of this dangerous complication in their case preparation should it arise.
Abbreviations
TAVI: transcatheter aortic valve implantation
AVS: symptomatic aortic valve stenosis
LM: left main
PCI: percutaneous coronary intervention
COPD: chronic obstructive pulmonary disease
CRF: chronic renal failure
LV: left ventricle
EF: ejection fraction
LCA: left coronary artery
LAD: left anterior descending artery
TEE: transesophageal echocardiography
DES: drug eluting stent
ICU: intensive care unit
LCX: left circumflex artery
CPR: cardiopulmonary resuscitation
LBBB: left bundle branch block
Introduction
Transcatheter aortic valve implantation (TAVI) is becoming a safe and effective technique for treating symptomatic aortic valvular stenosis (AVS) as an alternative to surgery in very high-risk patients1. An important step in this procedure with both commercially available devices is the correct positioning in respect to the aortic valve annulus. This is essential because, once deployed, it is not possible to reposition. A possible consequence of valve implantation is the obstruction of coronary ostia2.
Here we report on five cases of angiographically confirmed LM obstruction that occurred immediately after balloon expandable aortic valve implantations at our institution.
Case 1
In May 2008, an 86 year old female with a clinical history of hypertension, diabetes, COPD and CRF was admitted to the emergency room due to a pulmonary oedema. Echocardiography demonstrated the presence of a high grade of stenosis of a severely calcified aortic valve (jet velocity: 4.34 m/s; valve area index: 0.48 cm2/m2; mean pressure gradient: 51 mmHg) and a mild reduction of the LV systolic function (EF: 48%).
Coronary angiogram showed absence of coronary stenosis. The patient was referred for surgery. Due to her advanced age and the robust presence of comorbidities, her logistic EuroSCORE was calculated to be 24.25% and the patient was considered not a good surgical candidate and scheduled for a transfemoral TAVI.
At the time of TAVI, immediately after balloon valvuloplasty, an aortic valve calcification shifted towards the left main ostium causing a critical stenosis (Figure 1a and 1b).
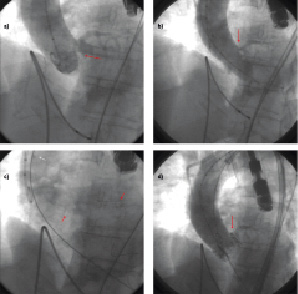
Figure 1. Left main obstruction following TAVI. a. Baseline aortography showing the presence of a bulky aortic leaflet calcification (red arrow). b. Aortography performed at the time of valvuloplasty, leaflet calcification is obstructing left main ostium (red arrow). c. A coronary guidewire is placed in the distal LAD (red arrows) and a guiding catheter is retracted in the ascending aorta (white arrow). d. Post TAVI aortography showing that the valve frame has shifted the aortic leaflet calcification that is now obstructing the coronary ostium (red arrow).
At this point, a radial artery access was obtained, an EBU 6 Fr guiding catheter (Medtronic, Minneapolis, MN, USA) was placed at the left main ostium and a BMW 0.014’’ coronary wire (Abbott Vascular, Redwood City, CA, USA) was advanced in the distal LAD. The guiding catheter was then retracted in the ascending aorta (Figure 1c) and a 26 mm Edwards SAPIEN aortic valve (Edwards Lifesciences, Irvine, CA, USA) was implanted at the aortic annulus, as confirmed by TEE evaluation.
Aortography showed persistent critical ostial LM stenosis (Figure 1d and 2a). Despite the presence of a TIMI 3 flow, hypotension and electrocardiographic evidence ischaemia immediately appeared. At this point, the guiding catheter was advanced at the ostial LCA and a drug eluting stent was directly implanted at the LM ostium (Figure 2b). A non-compliant balloon was use to post-dilate the ostium (Figure 2c).
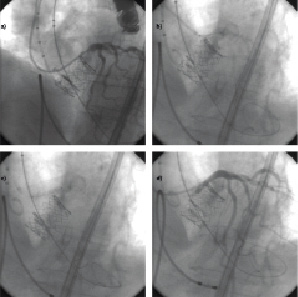
Figure 2. Management of left main obstruction following TAVI. a. Selective coronary angiography demonstrates the presence of a LM stenosis. b. A coronary stent is placed and deployed at the LM ostium. c. In-stent balloon post-dilation is performed. d. Final coronary angiographic showed resolution of LM obstruction.
Final pictures showed appropriate placement of the aortic valve and good angiographic results of the left main stenting (Figure 2d). The patient was sent back to the ICU, in-hospital stay was free of complications and the patient discharged home at day four. At 18 months follow-up patient was in good general condition and had a NYHA I functional class.
Case 2
In July 2008, a 78 year old female with a clinical history of hypertension, diabetes, COPD, CRF and previous CABG underwent TAVI due to the presence of an AVS (jet velocity: 4.26 m/s; valve area index: 0.51 cm2/m2; mean pressure gradient 46 mmHg) and concomitant elevated logistic EuroSCORE (51,47%). At the time of transapical implantation of a 23 mm Edwards SAPIEN aortic valve immediately after balloon valvuloplasty, an aortic valve calcification shifted towards the left main ostium causing a sub-occlusive stenosis (TIMI 1). Ventricular fibrillation occurred almost immediately. At this point, a brachial arterial access was obtained, an EBU 6 Fr guiding catheter (Medtronic, Minneapolis, MN, USA) was placed at the left main ostium and a BMW 0,014’’ coronary wire (Abbott Vascular, Redwood City, CA, USA) was advanced in the distal CX. Due to the urgency of this condition, mechanical assistance could not be placed and the procedure was performed during cardiopulmonary resuscitation (CPR). A predilation, with a compliant balloon, of the LM-CX and subsequent bare metal stent implantation were performed. Despite a good angiographic result and a TEE confirmation that the valve was correctly placed at the aortic annulus, cardiac function never recovered and the patient died of cardiac arrest.
Case 3
In October 2008, an 80 year old female with a clinical history of hypertension, diabetes, COPD and CRF underwent TAVI due to the presence of an AVS (jet velocity: 5.12 m/s; valve area index: 0.43 cm2/m2; mean gradient 58 mmHg) and concomitant high surgical risk (EuroSCORE 25.25%).
Immediately after a transapical 23 mm Edwards SAPIEN aortic valve implantation, a displaced bulky calcific leaflet caused abrupt occlusion (TIMI 0) of the left main ostium, with consequent haemodynamic comprise and cardiac arrest (Figure 3a). At this point, a brachial arterial access was obtained, an EBU 6 Fr guiding catheter (Medtronic, Minneapolis, MN, USA) was placed at the left main ostium and a BMW 0,014’’ coronary wire (Abbott Vascular, Redwood City, CA, USA) was advanced in the distal LAD under CPR (Figure 3b). After direct LM implant of a bare metal stent (Figure 3c) and post-dilation, with a non-compliant balloon, sinus rhythm was restored and cardiac function recovered. Final pictures showed appropriate placement of the aortic valve and good angiographic results of the left main stenting (Figure 3d). TEE confirmed that the valve was correctly placed at the aortic annulus and patient was sent back to the ICU. In-hospital stay was free of complication and patient discharged home at day five. At 12 months follow-up the patient was in good general condition and had a NYHA I functional class.
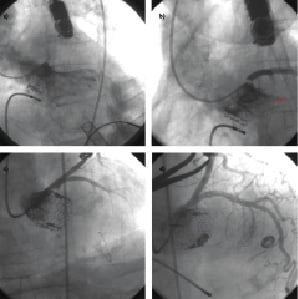
Figure 3. Complete left main obstruction following TAVI. a. Selective coronary angiogram showing complete left main obstruction following valve deployment. b. Minimal antegrade perfusion following placement of a coronary wire in the distal LAD (Red arrow). c. Coronary angiography, performed after left main stenting, during CPR. d. Coronary angiography, performed after stent post-dilation, showed complete resolution of left main obstruction.
Case 4
In November 2008, an 88 year old female with an AVS (jet velocity: 4.96 m/s; valve area index: 0.39 cm2/m2; mean gradient 55 mmHg) underwent TAVI due to the presence of high surgical risk (EuroSCORE 22%).
At the time of TAVI, aortography, performed at the time of valvuloplasty, showed the presence of a bulky aortic leaflet calcification shifted towards the sinotubular junction (Figure 4a). Aortography, performed after 23 mm Edwards SAPIEN aortic valve implantation, showed that the valve frame shifted the aortic leaflet calcification towards the sinotubular junction causing an obstruction of the left main ostium (Figure 4b). Selective coronary angiogram showed obstruction of LM ostium (Figure 4c) (TIMI 3). Hypotension and a new LBBB immediately appeared.
At this point, a femoral artery access was obtained and EBU 6 Fr guiding catheter (Medtronic, Minneapolis, MN, USA) was positioned at the LM ostium. After that two BMW 0.014’’ coronary wire (Abbott Vascular, Redwood City, CA, USA) were respectively advanced in the distal LAD and in the distal CX, a predilation with non-compliant balloon was performed. Due to the presence of a residual stenosis, a bare metal stent was implanted. Final pictures showed appropriate placement of the aortic valve and good angiographic results of the left main stenting (Figure 4d). TEE confirmed that the valve was correctly placed at the aortic annulus and patient was sent back to the ICU. In hospital stay was free of complication and patient discharged at day four. At 12 months follow-up, the patient had still a NYHA I functional class.
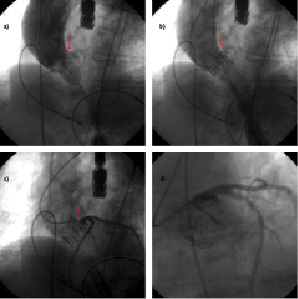
Figure 4. Left main occlusion following TAVI. a. Aortography, performed at the time of valvuloplasty, showing the presence of a bulky aortic leaflet calcification shifted towards the wall of the ascending aorta (red arrow). b. Aortography, performed after valve deployment, showing that the valve frame has shifted the aortic leaflet calcification towards the wall of the ascending aorta and is now obstructing the left main ostium (red arrow). c. Selective coronary angiogram showing left main obstruction (red arrows). d. Selective coronary angiogram, performed post left main PCI, showing resolution of left main obstruction and a good angiographic result.
Case 5
In March 2009 an 82 year old male underwent TAVI due to an AVS (jet velocity: 4.56 m/s; valve area index: 0.56 cm2/m2; mean gradient 43 mmHg) and a concomitant high surgical risk (20.7%). At the time of TAVI, aortography performed during balloon valvuloplasty, showed the presence of a bulky leaflet calcification shifted towards the sinotubular junction causing transient left main ostium stenosis (Figures 5a and 5b).
At this point, a brachial arterial access was obtained, an EBU 6 Fr guiding catheter (Medtronic, Minneapolis, MN, USA) was placed at the left main ostium and a BMW 0.014’’ coronary wire (Abbott Vascular, Redwood City, CA, USA) was advanced in the distal LAD.
The guiding catheter was then retracted in the ascending aorta (Figure 5c). A 23 mm Edwards SAPIEN aortic valve was implanted at the aortic annulus using a transapical approach. Aortic angiograms showed absence of LM stenosis (Figure 5d) and the wire was removed from the LAD. The patient was sent back to the ward. In-hospital stay was free of complications and the patient was discharged at day three. At eight months follow-up the patient was in good general condition and had a NYHA I class.
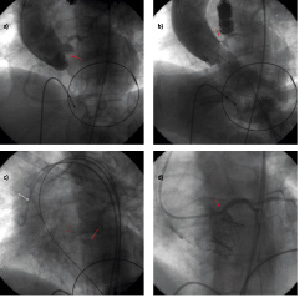
Figure 5. Transient left main obstruction following TAVI. a. Aortography showing the presence of a bulky aortic leaflet calcification (red arrow). b. Aortography performed at the time of valvuloplasty, leaflet calcification is obstructing left main ostium (red arrow). c. A coronary guidewire is placed in the distal LAD (red arrows) and a guiding catheter is retracted in the ascending aorta (white arrow). d. Post TAVI aortic angiogram showing the aortic leaflet calcification has not been shifted towards the left main ostium, which is well patent.
Discussion
These cases illustrate that obstruction of coronary ostia is a possible complication of TAVI.
Although LM occlusion was reported in animal studies, it is not commonly seen in patients undergoing percutaneous aortic valve replacement3. In an initial series of 18 patients treated with a transfemoral approach, reported by Webb et al, there was a fatal partial obstruction of left main ostium discovered only at autopsy evaluation4. In the initial report of 50 patients treated with a transapical approach, reported by Walther et al, an early conversion to conventional sternotomy had to be performed in a patient because of a coronary occlusion5. No events occurred in other registries6,7. Data from clinical trials have been reported at major meetings8. The database, including REVIVE, REVIVAL, TRAVERCE and PARTNER EU trials, showed an incidence of 0.4% coronary obstructions. In the SOURCE registry, an overall 0.6% incidence of coronary obstruction has been reported.
In our experience we observed a 4.1% risk of coronary occlusions (four patients had a persistent left main obstruction out of a group of 98 patients who underwent TAVI at our institution so far).
These events occurred at different time points of our learning curve, after we had been judged to be able to perform TAVI without company supervision.
The reasons for coronary artery compromise during TAVI could be different, and related to patient anatomy (bulky calcifications that can be displaced at the coronary ostia, low lying coronary artery, narrow and short sinus of Valsalva) or procedural events (valve misplacement or coronary emboli).
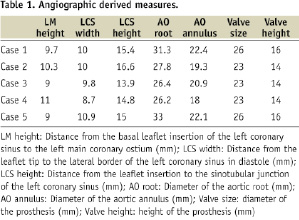
Few reports have discussed which patients can be considered at risk of coronary occlusion and several patterns have been suggested8: a low lying coronary ostium (<10–12 mm from the basal leaflet insertion to coronary ostium)9, bulky native leaflets, a narrow root with shallow sinuses of Valsalva leaving little room for the native leaflets. All of these can be assessed by echo, aortography, and CT and can be further assessed during valvuloplasty or even further by aortic root injection during valvuloplasty.
In particular, we suggest that if the aortography will show a stenosis of the LM ostium then it would be advisable to place a coronary wire in the distal LAD so as to be ready to face this complication if it occurs. It is important to remember that there may be a risk of prosthesis deformation at the time of device tracking (i.e., balloons, stents) if the first guidewire is trapped under a prosthesis strut. In this case, it would be advisable to advance a new working guidewire in the LM using the first wire as a road map.
Although surgical rescue can be planned in this type of emergency situation, it is not an optimal solution in these high risk patients. Appropriate interventional cardiology skills and set-up is critical to gain a favourable outcome in these cases. In this setting, it is crucial to consider the use of mechanical assisting devices (IABP, ECMO) that must be available and ready to be placed in function during these procedures.
Taking into consideration the experience derived from the management of these complications, we would suggest always having double arterial access during percutaneous aortic valve implantation in order to perform selective coronary angiography, as well as aortography. Moreover, a transfemoral venous access would be useful in order to perform cardiac assist if needed.
Although most of these cases were successfully managed, the possibility of contraindicating the procedure always has to be considered in the presence of high risk preprocedural features, or if balloon aortic valvuloplasty shows the potential of occlusion of the left main. As the use of TAVI becomes widespread, the operators should be aware of this dangerous possible complication in their case preparation should it arise.
Online movie legends
Video 1 (Case 1). Left main obstruction following TAVI. a) Baseline aortography showing the presence of a bulky aortic leaflet calcification. b) Aortography performed at the time of valvulopasty, leaflet calcification is obstructing left main ostium. c) A coronary guidewire is placed in the distal LAD and a guiding catheter is retracted in the ascending aorta. d) Post TAVI aortography showing that the valve frame has shifted the aortic leaflet calcification, that is now obstructing the coronary ostium.
Video 2 (Case 1). Management of left main obstruction following TAVI. a) Selective coronary angiography demonstrates the presence of a thight LM stenosis. b) A coronary stent is placed and deployed at the LM ostium. c) In-stent balloon postdilation is performed. d) Final coronary angiographic showed resolution of left main obstruction.
Video 3 (Case 3). Complete left main obstruction following TAVI. a) Selective coronary angiogram showing complete left main obstruction following valve deployment. b) Minimal antegrade perfusion following placement of a coronary wire in the distal LAD. c) Coronary angiography, performed after left main stenting, during CPR. d) Coronary angiography, performed after stent postdilation, showed complete resolution of left main obstruction.
Video 4 (Case 4). Left main occlusion following TAVI. a) Aortography, performed at the time of valvuloplasty, showing the presence of a bulky aortic leaflet calcification shifted towards the wall of the ascending aorta. b) Aortography, performed after valve deployment, showing that the valve frame has shifted the aortic leaflet calcification towards the wall of the ascending aorta and is now obstructing the left main ostium. c) Selective coronary angiogram showing left main obstruction. d) Selective coronary angiogram, performed post left main PCI, showing resolution of left main obstruction and a good angiographic result.
Video 5 (Case 5). Transient left main obstruction following TAVI. a) Aortography showing the presence of a bulky aortic leaflet calcification. b) Aortography performed at the time of valvulopasty, leaflet calcification is obstructing left main ostium. c) A coronary guidewire is placed in the distal LAD and a guiding catheter is retracted in the ascending aorta. d) Post TAVI aortic angiogram showing the aortic leaflet calcification has not been shifted towards the left main ostium, that is well patent.

