Abstract
Aims: Coronary protection with guidewires and an undeployed coronary balloon or stent positioned in the coronary artery is a pre-emptive technique to manage coronary obstruction during transcatheter aortic valve implantation (TAVI). We investigated the feasibility and safety of left main (LM) protection during TAVI.
Methods and results: Twenty-five out of 623 patients who underwent TAVI at our institute were deemed to be at increased risk of LM compromise mainly due to a low LM ostium height, significant LM disease or a previous bioprosthetic valve. A pre-emptive LM protection technique was therefore used in these cases. Five patients (20%) had pre-TAVI significant non-revascularised LM stenosis, and four patients (16%) had a prior LM ostial stent without pre-TAVI in-stent restenosis. Twelve patients had extremely low LM height (mean height 6.7±2.4 mm; range 1.1-8.9 mm). Seven patients (25%) had valve-in-valve (VIV) procedures. LM compromise occurred in five out of 25 cases; all were treated successfully with emergency LM stenting. Nine patients underwent successful planned LM procedures following TAVI.
Conclusions: The LM protection technique should be considered in patients deemed to be at increased risk of LM compromise. This was found to be helpful in the prompt diagnosis and treatment of LM compromise following TAVI.
Abbreviations
AS: aortic stenosis
CAD: coronary artery disease
IVUS: intravascular ultrasound
LAD: left anterior descending
LM: left main
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
SOV: sinuses of Valsalva
STJ: sinotubular junction
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
VIV: valve-in-valve
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as a treatment option for inoperable or high-risk surgical patients with severe aortic stenosis (AS)1. Coronary obstruction, defined as a new, partial or complete obstruction of a coronary ostium during or after the procedure, is a rare but life-threatening complication of TAVI2,3. The reported incidence of coronary obstruction is 0.8%, ranging from 0% to 4.1% in contemporary series4-6.
Coronary obstruction is usually caused by displacement of the calcified native valve leaflet over the coronary ostium or by the direct occlusion of the coronary ostium by the covered skirt of the transcatheter aortic prosthesis. It is associated with anatomical factors including lower-lying coronary ostia and shallow sinuses of Valsalva (SOV), and with valve-in-valve (VIV) for surgical bioprosthesis5,7. The 30-day mortality rate of coronary obstruction following TAVI was found to be as high as 41% in a recent large multicentre registry analysis6, which highlights the importance of anticipating and preventing the occurrence of this complication.
Coronary protection with a guidewire, coronary balloon or stent, positioned in the coronary artery, is a pre-emptive technique to manage coronary obstruction during TAVI. This technique is guided by preprocedural imaging and contingency planning and may help in the early diagnosis and treatment of coronary compromise following valve deployment. It may also facilitate rapid planned LM stenting when significant LM disease and severe aortic stenosis coexist. Reported clinical experience with this technique is limited, merely in the form of case reports8. Here, we describe the largest single-centre experience of systematic LM coronary protection during TAVI.
Methods
Of 623 consecutive patients undergoing TAVI from April 2012 to May 2014 at our institute, LM coronary protection was used in 25 patients deemed to be at increased risk of LM compromise. The indication for TAVI was severe AS in 23 patients and severe aortic regurgitation in two patients. All patients had congestive heart failure with New York Heart Association (NYHA) Class III or IV symptoms. All underwent preprocedural coronary angiography to assess the need for revascularisation. Aortic valve disease was assessed initially with transthoracic echocardiography followed by an ECG-gated, multislice CT angiography study with a SOMATOM Sensation 64 scanner (Siemens Medical Solutions USA, Inc., Malvern, PA, USA). CT analysis included aortic annulus diameter and area, coronary height, SOV diameter, sinotubular junction (STJ) diameter, severity of aortic valve calcification, presence of aortic valve calcium nodules (>10 mm), prosthesis size/annulus diameter ratio and prosthesis area/annulus area ratio. TAVI was performed under general anaesthesia in all cases, utilising the Edwards SAPIEN/XT/S3 valve (Edwards Lifesciences, Irvine, CA, USA) in 21 patients or the CoreValve® (Medtronic, Minneapolis, MN, USA) in four patients. The approach was chosen on the basis of the individual patient’s risk profile, being transfemoral (20 patients), transaortic (three patients) or transapical (two patients). All patients were considered high risk for valve surgery by our institutional Heart Team. Baseline clinical, echocardiographic and procedural details of TAVI were recorded for all patients including one-month clinical and echocardiographic assessments during a follow-up visit. TAVI endpoints, device success and adverse events were considered according to the Valve Academic Research Consortium (VARC)-2 definitions3.
LM CORONARY PROTECTION
INDICATIONS FOR LM PROTECTION
After a thorough review of each patient’s clinical profile, preprocedural angiography, CT and echocardiography, pre-emptive LM coronary protection was used primarily for three reasons. 1) Anatomical features: the LM ostium height was defined as the distance between the LM ostium and the aortic annulus as measured by CT scan (based on curved multiplanar reconstructions; CMPR). We usually considered an LM height of less than 9 mm as an indication for LM protection. A difference of less than 2 mm between the SOV mean diameter and the prosthesis diameter or severe aortic valve calcifications with the presence of left cusp large bulky calcium nodule(s) also indicated LM protection. 2) LM significant disease defined as ≥50% angiographic stenosis and intravascular ultrasound (IVUS) minimal luminal area of <6 mm² or a previous LM ostial stent. 3) Previous bioprosthetic valve – for VIV procedures we used LM protection in cases of stentless valves (including homograft that has no stent frame), and pericardial surgical heart valve with leaflets sutured outside the stent (Mitroflow; Sorin Group Inc., Milan, Italy, or Trifecta™; St. Jude Medical, St. Paul, MN, USA) because the leaflets of these valves may extend outwards in a tubular fashion following TAVI and cause coronary compromise7,9. Additionally, coronary protection was performed in VIV cases with high-risk anatomical features for coronary obstruction, as described above.
METHOD OF LM PROTECTION
For each case of LM protection we used a 6 Fr XBLAD guiding catheter (Cordis, Johnson & Johnson, Warren, NJ, USA) advanced through the contralateral femoral artery and positioned at the LM ostium, and one or two Balance Middleweight (BMW; Abbott Vascular, Santa Clara, CA, USA) 0.014” coronary wires advanced to the distal left anterior descending (LAD) and circumflex arteries. We preferred to use two wires in order to have additional support in case LM stenting was needed. In case of a short ostial LM lesion not extended into the bifurcation, one wire may be equally effective and an additional wire may be advanced if needed. LM protection for self-expanding valves has the notable difference that the guiding catheter and coronary wires are located behind rather than above the longer bioprosthetic stent frame (Figure 1). In order to protect the LM further, in some patients an undeployed coronary balloon or stent was positioned in the proximal LAD prior to the beginning of valve deployment in preparation for emergent or planned usage following TAVI (Figure 1). Immediately following valve deployment a selective angiography was performed to assess partial or complete obstruction following the procedure. Planned stenting immediately following valve deployment was performed in cases of pre-TAVI significant LM stenosis as defined above. Planned balloon inflation was also performed immediately after TAVI in all previous ostial LM stents in case the stent struts were disrupted by balloon inflation during valve deployment or during predilatation or post-dilatation. Emergency LM stenting was performed in cases of any symptomatic or asymptomatic new, partial significant or complete LM obstruction (Figure 2, Moving image 1). When an undeployed stent was positioned in the LAD artery, ready to be pulled proximally into the LM in case of obstruction, the shortest available stent (12 mm) was used in order to avoid unnecessary LM bifurcation stenting. Drug-eluting stents were the preferred stent type in the absence of a contraindication for a long-term dual antiplatelet therapy. In special scenarios, bare metal stents were used due to their increased radial strength. For instance, in one patient in our series, a drug-eluting stent was deployed for coronary obstruction following transcatheter heart valve deployment. The stent was noted to be underexpanded due to impingement by the stent frame. A bare metal stent was subsequently deployed due to the increased radial strength with resultant adequate expansion and lesion coverage.
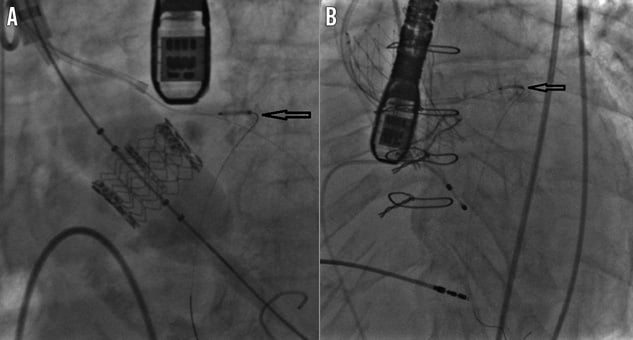
Figure 1. LM protection method. A) LM protection during balloon-expandable TAVI using guiding catheter, two guidewires and an undeployed drug-eluting stent (arrow). B) LM protection during self-expanding TAVI using guiding catheter and guidewire located behind rather than above the longer bioprosthetic stent frame. An undeployed drug-eluting stent is located in the proximal LAD artery (arrow).
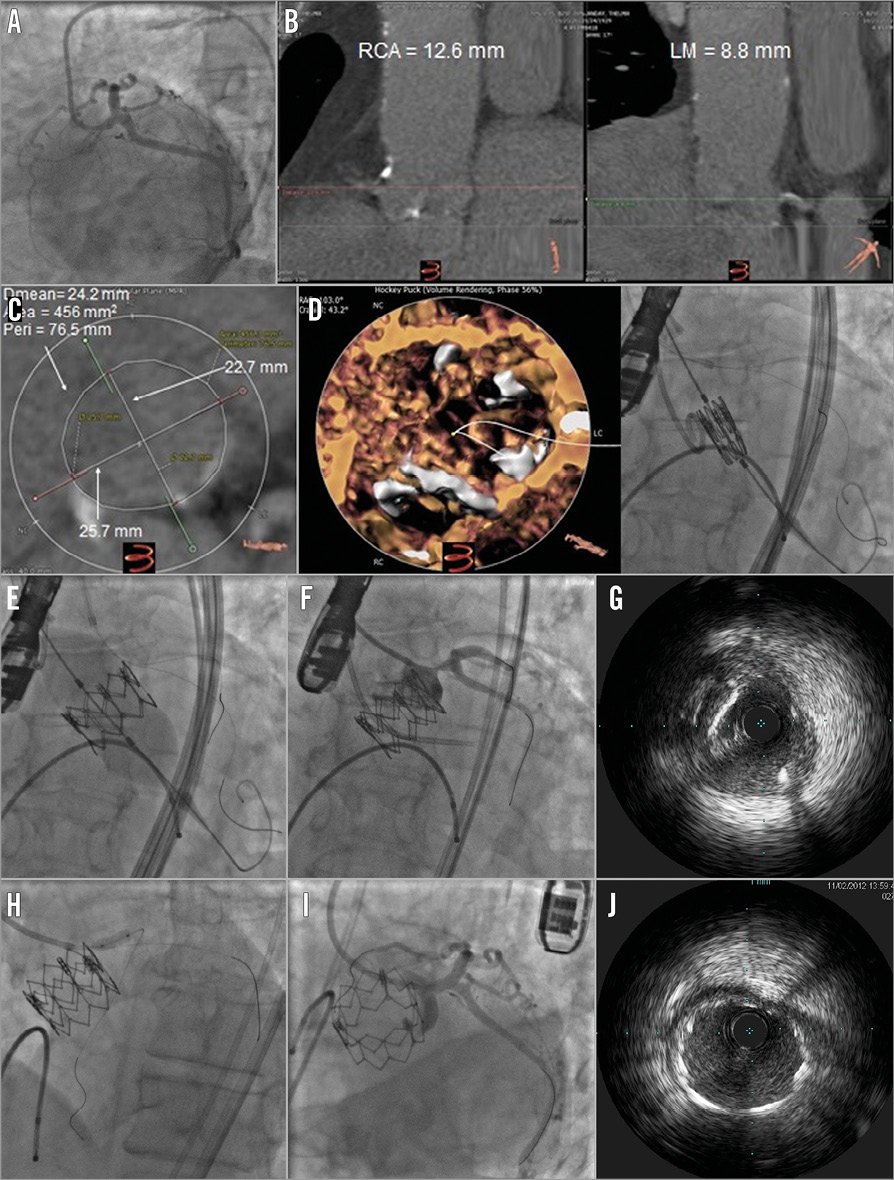
Figure 2. Case example of LM protection technique during balloon-expandable TAVI. A) Pre-TAVI left coronary angiography showing no significant CAD in the LM. B) Preprocedural CT analysis of coronary height revealing a low-lying LM ostium. C) Preprocedural aortic valve analysis showing axial view of the valve annulus sizing suitable for a 26 mm Edwards SAPIEN XT valve. D) LM protection using a 6 Fr XBLAD guiding catheter and two guidewires advanced to the distal LAD and circumflex arteries before valve deployment. E) Traction of the guiding catheter just before valve deployment preventing catheter compression by the expanded valve frame (Moving image 1). F) Routine selective injection to the left coronary artery post valve deployment, showing a new ostial LM 80% stenosis. G) IVUS showing fibrocalcific plaque in the ostium of the LM with a minimal luminal diameter of 4.2 mm. H) Deployment of a 3.5x12 mm everolimus-eluting stent (XIENCE; Abbott Vascular, Santa Clara, CA, USA) in the LM. I) Final angiogram demonstrating TIMI 3 flow with no significant stenosis in the LM. J) IVUS showing well-expanded LM stent with no residual stenosis.
STATISTICAL ANALYSIS
Due to the observational, single-arm basis of this registry, only descriptive statistical analysis has been performed. Data are presented as mean±standard deviation if continuous, or as a number (percentage) if dichotomous.
Results
Of 623 patients who underwent the TAVI procedure at our institute, we used pre-emptive LM protection in 25 cases (4.01%). The clinical and TAVI procedural characteristics of the study population are shown in Table 1. Mean age was 79.5 years and 52% of patients were female. Sixteen patients (64%) had a previous history of coronary artery disease (CAD). Pre-TAVI CT scans were performed and analysed in 24 patients and are presented in Table 2.
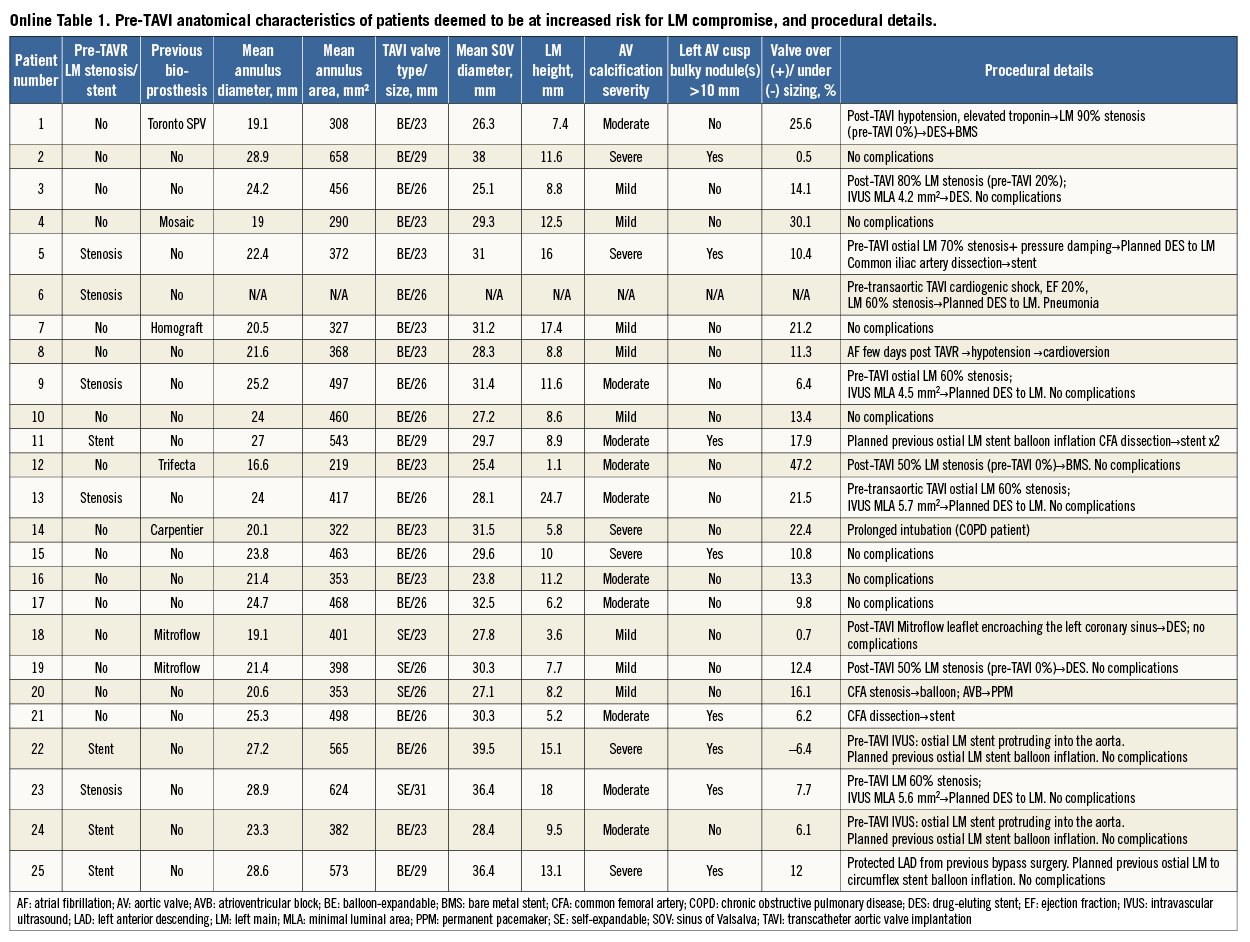
According to the pre-TAVI evaluation, 25 patients were deemed to be at increased risk of LM compromise (Online Table 1). In 11 cases, the primary indication for LM protection was based on anatomical high-risk features: seven patients had extremely low LM height (mean height 7.4±1.6 mm; range 1.1 to 8.8 mm), one patient had 23.8 mm mean SOV diameter and a 23 mm valve implanted, two patients had severe aortic valve calcifications with large (>10 mm), bulky calcium nodule(s) in the left cusp, and one patient had extreme (30%) valve oversizing - all four patients had concomitant relatively low LM (10-12.5 mm). Five other patients with extremely low LM height had another primary indication for LM protection - four had a previous bioprosthesis with a high risk of LM compromise and one had LM disease. Five patients (20%) had pre-TAVI significant non-revascularised LM stenosis ranging from 60 to 70%, and four patients (16%) had a prior LM ostial stent without pre-TAVI in-stent restenosis. From the 29 VIV procedures performed at our institute during the study period, LM protection was used in seven patients –five of them had a bioprosthesis with high risk for LM compromise: two had a Mitroflow valve, one had a Trifecta valve and two had stentless valves (Toronto SPV [stentless porcine valve]; St. Jude Medical, St. Paul, MN, USA, and human homograft). In the remaining two cases, additional anatomical high-risk features were the primary indication for LM protection, one case of extremely low LM and one case of extreme (30%) oversizing.
LM PROTECTION METHOD AND NEED FOR INTERVENTION
An XBLAD guiding catheter was used in all cases: one or two coronary guidewires were used in five and 20 cases, respectively. An undeployed coronary angioplasty balloon placed in the LAD during the TAVI implant was used in seven cases and an undeployed coronary stent placed in the LAD in eight. LM compromise occurred in five out of 25 cases (20%). Of these five patients, three patients underwent TAVI with balloon-expandable Edwards valves and two underwent TAVI with self-expanding Medtronic CoreValve. Only one of them had clinical signs (acute hypotension and diffuse ST depression on the electrocardiographic monitor); the others were found during selective LM injections following valve deployment. In four of these cases, there was a 50 to 90% new LM stenosis and in one case of VIV procedure a Mitroflow leaflet seemed to be displaced close to the LM ostium, immediately following valve deployment, thus jeopardising LM flow. These patients underwent unplanned emergency LM stenting to treat the de novo LM compromise that was noted after valve deployment. Four patients underwent planned balloon inflation in a previously placed LM ostial stent, and five patients with pre-TAVI significant LM stenosis underwent planned LM stenting following valve deployment, all resulting in TIMI 3 flow without any complication. A total of 11 stents were used to treat the LM in 10 patients - nine of these were drug-eluting stents and two were bare metal stents. In one case an IVUS performed after stent deployment revealed incomplete ostial LM coverage with residual stenosis and therefore a second stent with higher radial force was used. A summary of the indications for LM protection, procedural details and outcome is presented in Table 3. In the 598 patients who were not treated with the pre-emptive LM protection during TAVI, there was one case of LM obstruction during TAVI (0.17%). This patient’s preprocedural CT scan revealed an LM height of 19.6 mm, a relatively large SOV (38.1 mm) and moderate aortic valve calcification. Following deployment of a 29 mm SAPIEN XT valve (Edwards Lifesciences) he was noted to be hypotensive, and a new significant LM stenosis was demonstrated on the coronary angiogram. He was treated successfully with drug-eluting stent deployment to the ostial LM and was alive at two-year follow-up.
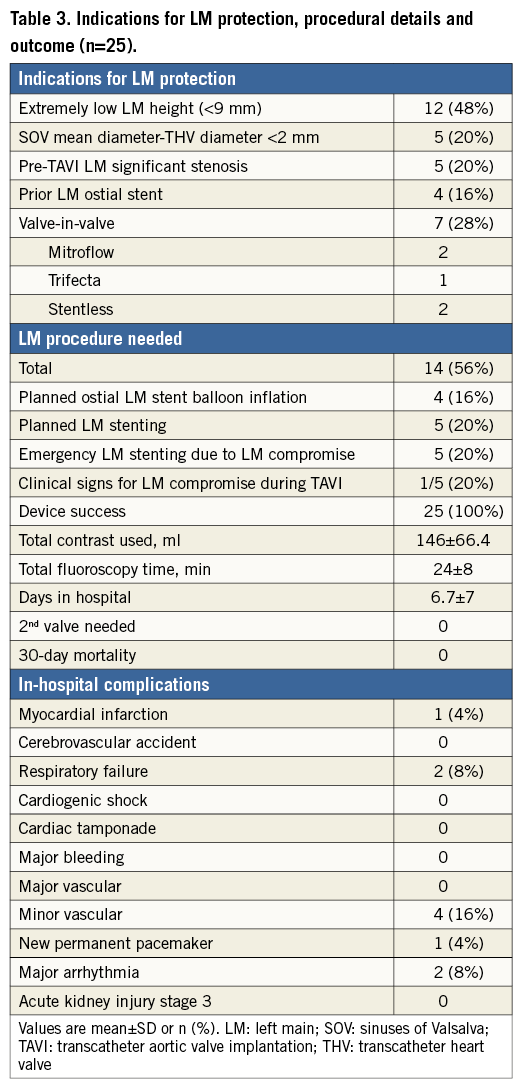
TAVI CLINICAL OUTCOME
Device success was 100% with no need for a second valve in any case. There was no case of in-hospital mortality, cardiac tamponade, acute kidney injury stage 3 or cerebrovascular accident (Table 3). There was one case of periprocedural myocardial infarction in a patient who developed hypotension and was found to have 90% LM stenosis followed by significant troponin elevation. There was one case of atrial fibrillation and hypotension a few days after TAVI which was treated successfully with direct current cardioversion and medical therapy. There was one case of prolonged hospitalisation secondary to pneumonia and one delayed extubation in a patient with COPD. There were two cases (8%) of mortality at follow-up ranging from two to 28 months (mean follow-up 11.5±7.8 months). These patients died from sepsis and sudden cardiac death two and 10 months after the procedure, respectively. Among the 598 patients who did not undergo coronary protection there were three cases (0.5%) of periprocedural myocardial infarction (one with LM obstruction as described above), 15 cases of in-hospital cerebrovascular accident (2.5%), and three cases of cardiac tamponade (0.5%). Thirty-day mortality was 3% (18 patients) and overall mortality during the follow-up period was 14.7% (88 patients).
Discussion
The results of the present study indicate that performing pre-emptive LM protection in selected patients deemed to be at increased risk of LM compromise is feasible and safe. Implementing this technique resulted in performing LM procedures in 14 out of 25 patients (56%), five of them (20%) unplanned procedures for angiographic coronary compromise. There was no case of complication directly related to the usage of guiding catheter, guidewires, coronary balloon or stent, and no case of 30-day mortality. We also found that the majority of LM compromise cases were without clinical signs during the procedure and were detected during routine selective LM contrast injection post valve deployment.
Coronary obstruction is a rare but life-threatening complication of TAVI with a reported incidence of <1% which carries a high mortality when it occurs4,6. In cases of VIV implantation, the risk of coronary obstruction is significantly higher (3.5% in the global VIV registry)7. In a recent large multicentre registry evaluating coronary compromise after TAVI, 44 patients out of 6,688 (0.7%) suffered symptomatic coronary obstruction6. Percutaneous coronary intervention (PCI) was attempted in 75% of the cases and was unsuccessful in 18%, while 30-day mortality was 41% overall. Left coronary artery obstruction was found to be substantially more common than right coronary artery obstruction5,6.
Thorough evaluation of each patient before TAVI, using coronary angiography, CT scan and echocardiography, enabled us to identify patients we believed to be at increased risk for LM compromise during TAVI. The utilisation of the coronary protection method helped in early diagnosis and efficient treatment of LM compromise following TAVI. We implemented the coronary protection method for three main indications: (i) anatomical risk factors for LM compromise, (ii) significant coexisting LM disease, and (iii) previous bioprosthetic valve with high-risk features.
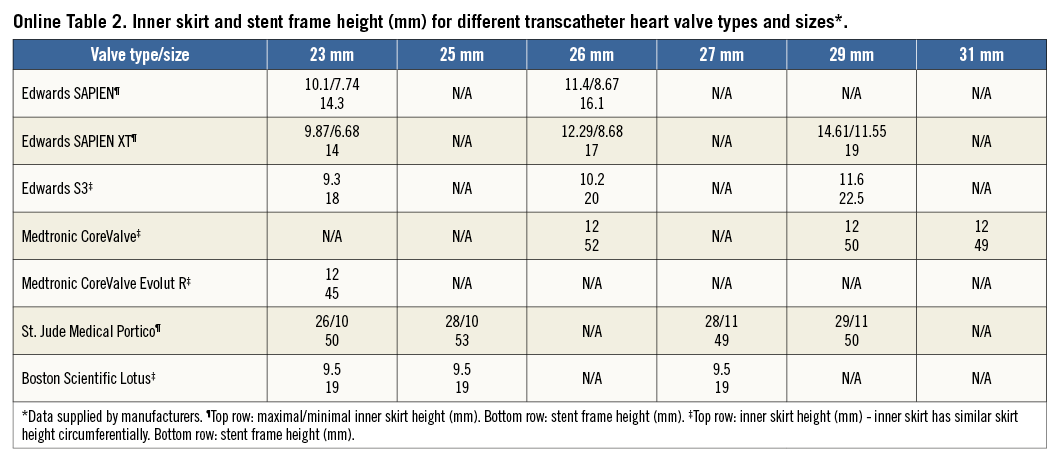
Several anatomical factors may influence the risk of coronary compromise during TAVI. Low LM ostium height was an important reason for LM protection in our study. Although coronary obstruction is usually caused by displacement of the calcified native valve leaflet over the coronary ostium, it is probably influenced more by anatomical factors than by the severity of valve calcification2,6,10. Previously, it has been suggested that a coronary ostium height cut-off of ≤10 mm increases the risk of coronary obstruction during TAVI11. Nonetheless, both a recent systematic review and a large multicentre registry reporting coronary obstruction cases suggested that a 12 mm cut-off is more relevant in predicting this complication5,6. We chose a cut-off of 9 mm for mandatory protection of extremely low LM and considered other anatomical factors in cases of borderline low LM (9-12 mm). Interestingly, the five cases of post-TAVI LM coronary compromise which resulted in an emergency LM procedure occurred only in patients with LM height of less than 9 mm. Moreover, when we retrospectively evaluated all cases and examined the height of the inner valve skirt in the different transcatheter heart valve (THV) types and sizes, we found that it varies substantially, and this can potentially influence the risk of coronary compromise (Online Table 2). It should be emphasised that other anatomical factors also contribute to coronary compromise during TAVI. These factors include shallow sinuses of Valsalva, severe aortic valve calcifications with large, bulky calcium nodules, high native leaflet length/curved coronary sinus height ratio and extreme oversizing of the new implanted valve allowing little room to accommodate the calcified native aortic valve leaflets after valve deployment2,5,6,10. A thorough evaluation of anatomical factors is therefore most important for the assessment of coronary obstruction risk in patients with borderline range of low LM heights (9-12 mm). It should also be highlighted that extreme oversizing should be avoided due to the potential risks of coronary obstruction and annulus rupture. However, in the case of extreme oversizing presented in our study there was no smaller valve available at that time and therefore a 23 mm SAPIEN valve was implanted in a degenerated bioprosthetic 23 mm Medtronic Mosaic valve. The safety of the coronary protection method presented herein should encourage a low threshold for utilisation of this technique in cases where concern is raised in preprocedural planning.
Nine of our patients (36%) had significant LM disease pre-TAVI (n=5) or a previous LM ostial stent without pre-TAVI in-stent restenosis (n=4). LM protection was used in these cases regardless of the coronary height. The rationale was that in cases of LM preprocedural stenosis even a small amount of calcium dislodgement might result in critical life-threatening LM compromise that can be managed more easily if guidewires and an undeployed stent are already in place.
CAD is often found concurrently in patients presenting with severe AS12. While surgical candidates with both diseases are treated with surgical aortic valve replacement combined with coronary artery bypass grafting, there is still no consensus on the management of severe CAD in patients referred for TAVI13. PCI before TAVI in a staged procedure is preferred by some14, while others perform TAVI with PCI as a combined procedure15. Performing staged PCI before TAVI offers the advantages of simplified access to the coronaries compared to accessing the coronaries in the presence of the transcatheter aortic valve, and a decreased risk of ischaemia and haemodynamic instability secondary to rapid pacing and balloon inflation performed during TAVI. Staging LM PCI followed by TAVI, especially in patients with chronic kidney disease, also offers the advantage of a decreased risk of contrast nephropathy, by facilitating contrast administration during two separate procedures13. Conversely, the need for uninterrupted dual antiplatelet therapy following stenting predisposes patients to an increased risk of bleeding, especially when TAVI is performed using a non-transfemoral approach (transaortic, transapical or subclavian approach). Moreover, there is a paucity of evidence on the safety of LM PCI in the presence of severe AS. Goel et al16 reported no difference in short-term mortality associated with PCI in patients with severe AS compared to those without severe AS. However, only 12% of the patients in the study had LM PCI, and the effect of LM stenting on mortality was not analysed. The potential advantages of the combined approach are treatment of both pathologies at the same time with elimination of potential morbidity and mortality after PCI while awaiting definitive management and arterial access for PCI and TAVI at the same time, with a potential reduction in the risk of vascular access-site complications and bleeding.
Patients with significant unprotected LM disease referred for TAVI present unique challenges regarding procedural safety. The only reported series describing the treatment of unprotected LM and concomitant TAVI included seven patients - in six of them PCI was performed prior to TAVI in a staged manner17. Five patients in our study underwent TAVI immediately followed by deployment of an LM coronary stent. The LM lesions in these patients were non-complex lesions located in the proximal segment of the LM, did not involve LM bifurcation and were free of significant calcification. Due to concern for significant anatomic interaction between the ostial LM stents and the transcatheter heart valve, the LM lesions in these patients were treated during TAVI, immediately following transcatheter valve deployment. Moreover, in two out of these five cases a transaortic approach was used, and dual antiplatelet therapy interruption prior to TAVI could have been avoided by performing a combined procedure. The induction of brief myocardial ischaemia during LM PCI or during rapid pacing and balloon inflation when the valve is deployed can potentially lead to acute haemodynamic decompensation. Thus, we elected to perform concomitant TAVI and LM PCI in patients deemed to be at risk of significant haemodynamic compromise during LM PCI in the presence of severe AS. Nonetheless, only scarce data regarding LM stenting and TAVI have been published, and the limited experience of concomitant TAVI and LM stenting reported in the present study needs to be confirmed in a larger study.
In the present study, all five patients with pre-TAVI significant LM stenosis underwent planned LM stenting immediately following valve deployment, resulting in TIMI 3 flow without any periprocedural major complications. We believe that performing a combined procedure with the utilisation of LM protection which enables prompt treatment of the diseased LM immediately following successful valve replacement is an effective means of minimising the risk of stent injury by transcatheter valve deployment. In the presence of complex LM lesion anatomy (e.g., distal bifurcation lesions, calcified lesions) when a longer procedure is expected, we recommend performing LM PCI with or without balloon aortic valvuloplasty, followed by TAVI in a staged manner.
With a similar rationale, in the other four cases of previous ostial LM stent without in-stent restenosis, the technique was employed to protect the struts of the stent which can be deformed during deployment of the valve’s metallic frame by the long balloon used to inflate balloon-expandable valves, or by external compression from the calcified native valve cusp. Kang et al18 examined IVUS of patients with proximal LM lesions treated with drug-eluting stents. Interestingly, 68% (156/229) of the stents used to treat proximal LM disease had protruded into the aorta (average length of stent protrusion: 3.4 mm). In cases of ostial LM disease, 94% (61/65) of the stents protruded into the aorta with 60% of the stents protruding more than 3 mm. In a recent autopsy study, TAVI was performed in 40 post-mortem hearts19. It was found that, following a properly implanted bioprosthesis, the native aortic leaflets were folded upward, pushed by the valve frame and immobilised against the leaflets close to the aortic wall. Most of the coronary ostia were partially or fully covered by the native leaflets. In order to prevent disruption of an ostial stent that may protrude into the aorta, balloon inflation in the ostial LM stent was performed successfully in four cases of previous ostial LM stents in our study immediately following valve deployment.
We used LM protection in seven out of 29 VIV procedures (24%) compared to 18 out of 594 native valve procedures (3%). The occurrence of LM obstruction is substantially more frequent among patients with previous surgical aortic bioprosthesis6,7. Its reported incidence of 2.4%-3.5% emphasises that in VIV procedures the risk of LM compromise should be evaluated thoroughly. Some types of surgical prosthesis have been associated with this complication6. Three out of the seven cases of coronary obstruction in the global VIV registry published by Dvir et al occurred in patients with a failed Mitroflow valve: this was attributed mainly to the long and externally mounted leaflets of this valve7. Pericardial surgical heart valves with leaflets sutured outside the stent (Mitroflow and Trifecta), as well as valves with no stent frame (stentless valves and homograft), are at higher risk of obstruction because their leaflets may extend outwards in a tubular fashion following VIV deployment, jeopardising the coronary flow8,9. The three commissural posts of a typical stented bioprosthesis may limit the outward displacement of the bioprosthetic leaflets and decrease the risk of coronary compromise. Extreme oversizing may cause outward deflection of these posts and minimise this protective effect and should therefore be avoided9. Another important anatomical factor that should be considered when evaluating patients with a bioprosthetic valve, which is often supra-annular, is that measurement of coronary height should be from the LM ostium to the bioprosthetic annulus and not to the native aortic annulus, since the former is the landing zone for the TAVI device rather than the latter. Supra-annular bioprosthetic valves may be stentless (e.g., St. Jude SPV Toronto valve; Sorin Freedom Solo valve) or stented (e.g., Carpentier-Edwards valve; Sorin Soprano valve and the Medtronic Mosaic and Mosaic Ultra valves).
Previous reports have demonstrated that most patients with coronary obstruction presented with complete obstruction, persistent hypotension and about one half of them exhibited electrocardiographic changes6,20. Interestingly, four out of the five patients with LM obstruction in the present study were without clinical signs, and partial significant LM stenosis was discovered during routine selective LM injection at the end of the procedure. Without routine selective LM injection, the diagnosis of LM compromise could have been significantly delayed, causing serious complications. Moreover, partial and not complete occlusion has occurred with the presence of a wire with or without an undeployed balloon or stent in the left main that could have contributed to vessel patency in itself. This also highlights the possibility that the incidence of coronary obstruction is probably higher than reported in the literature due to misdiagnosis. This argument is supported by data from the first series of patients treated with retrograde transfemoral TAVI (Webb et al), in which there was a fatal partial obstruction of the LM ostium discovered only at autopsy in a patient who seemingly died from pneumonia21.
Although some analyses show that coronary obstruction is more prevalent when using balloon-expandable compared to self-expanding valves, this may be the result of differences in manufacturer-directed preprocedural screening: a specific recommendation for minimal sinus of Valsalva width and coronary height is provided by the manufacturer for the CoreValve system4,6 but not for the balloon-expandable system. Different frame characteristics (Online Table 2) and mechanisms of implantation could also contribute to these differences. The present case series involved implantation of 21 balloon-expandable valves versus four self-expandable valves; however, this should be attributed solely to the fact that self-expandable valves were implanted at our institute only in the last four months of the study period.
Another measure that may be helpful in identifying patients at risk of LM obstruction is performing balloon predilatation with simultaneous aortography which demonstrates flow in the LM9. It can assist in recognising cases in which LM protection should be used or alternatively help in the decision for substitute treatment options such as redo surgery. It is our routine practice to perform TAVI without predilatation or with moderate predilation with smaller sized balloons instead of regular predilation in order to minimise manipulation of the calcified native valve. We therefore do not routinely employ the predilatation aortography technique to determine the risk of coronary compromise during TAVI.
It may be argued that TAVI is not the preferred treatment modality in patients with an extremely low LM ostial height, especially when associated with important valve calcification and/or shallow sinus of Valsalva. To the best of our knowledge, this is the first reported series of pre-emptive LM protection during TAVI. Previous descriptions of coronary protection during TAVI were in the form of case reports8. Based on our experience, we suggest a decision-making flow chart for the preprocedural evaluation of a patient believed to be at increased risk of LM compromise during TAVI (Figure 3).
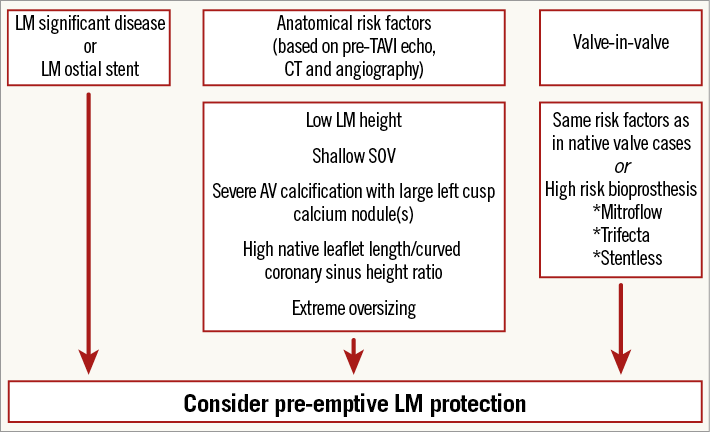
Figure 3. Suggested flow chart for pre-emptive LM protection based on pre-TAVI evaluation. AV: aortic valve; LM: left main; SOV: sinuses of Valsalva; TAVI: transcatheter aortic valve implantation
Study limitations
The present observational report summarises the experience of a single institution with only a limited number of patients undergoing TAVI with pre-emptive LM protection. Therefore, the results are exploratory and require confirmation in larger multicentre studies during longer-term follow-up with propensity-matched patient analysis. Nonetheless, the rates of complications are low and comparable with routine TAVI outcomes. The method of LM protection was different among patients, involving placement of an undeployed balloon or stent in some and guidewires only in others according to the operator’s decision. Although all methods were found to be safe in our report, the small number of patients prevents any conclusions concerning the preferred method.
Conclusions
Meticulous planning of the procedures deemed to be at increased risk of LM compromise, using thorough evaluation of coronary, aortic root and aortic valve anatomy, and employing coronary protection, resulted in five emergency and nine planned LM procedures out of 25 cases without cardiogenic shock, coronary perforation, cardiac tamponade, acute kidney injury type 3 or mortality. The results of this present study should encourage utilisation of coronary protection in appropriately selected patients as described above.
| Impact on daily practice Coronary protection with a guidewire, coronary balloon or stent positioned in the coronary artery is a pre-emptive technique to manage coronary obstruction during TAVI. This technique is guided by preprocedural imaging and contingency planning and may help in the early diagnosis and treatment of coronary compromise following valve deployment. Placing of a protective guidewire in the left coronary artery enables rapid revascularisation in case of LM compromise, and an undeployed balloon or stent further simplifies prompt procedure if emergently needed. Performing pre-emptive LM protection in selected patients deemed to be at increased risk of LM compromise is feasible and safe. |
Conflict of interest statement
R. Makkar has received grant support from Edwards Lifesciences and St. Jude Medical, is a consultant for Abbott Vascular, Cordis, and Medtronic, and holds equity in Entourage Medical. H. Jilaihawi is a consultant for Edwards Lifesciences, St. Jude Medical, and Venus MedTech. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Traction of the guiding catheter just before valve deployment preventing catheter compression by the expanded valve frame.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Traction of the guiding catheter just before valve deployment preventing catheter compression by the expanded valve frame.

