CASE SUMMARY
BACKGROUND: An 86-year-old man with an aortic valve stenosis who successfully underwent a Medtronic CoreValve percutaneous valve implantation after ostial left main stenting.
INVESTIGATION: Physical examination, electrocardiogram, coronary angiogram, CT scan.
DIAGNOSIS: Aortic valve stenosis, ostial subocclusive stenosis of the left main.
MANAGEMENT: Left main stenting, transcatheter aortic valve implantation (TAVI).
KEYWORDS: left main coronary, percutaneous coronary intervention, transcatheter aortic valve implantation
PRESENTATION OF THE CASE
An 86-year-old man with a calcified aortic valve stenosis (AVS) (valve area of 0.75 cm2 with a peak valve gradient of 85 mmHg and a mean valve gradient of 51 mmHg), and symptoms of dyspnoea with minimal activity (NYHA Class III), having already received a stent implant to the circumflex artery, was hospitalised in our department. The echocardiogram confirmed the severity of the aortic stenosis and showed preserved left ventricular systolic function (ejection fraction=58%). Considering the patient’s age and the high surgical risk (logistic EuroSCORE: 25.4%), the patient underwent a screening for a transcatheter aortic valve implantation (TAVI). The coronary angiography showed: focal and ostial subocclusive stenosis of the left main (LM) (Figure 1), occlusion of the non-dominant right coronary artery, as well as the patency of a stent previously implanted to the circumflex artery. During the angiography, the patient began to experience chest pain; systemic arterial pressure decreased suddenly, and the electrocardiogram showed pronounced ST-T segment depression (Figure 2). Therefore, the patient underwent an emergency coronary angioplasty with the implant of a 3.5×12 mm bare metal stent on the ostial LM (Moving image 1). Immediately after, the clinical status rapidly improved with the ceasing of pain, the subsiding of the ST-T segment deviations, and the recovery of arterial pressure.
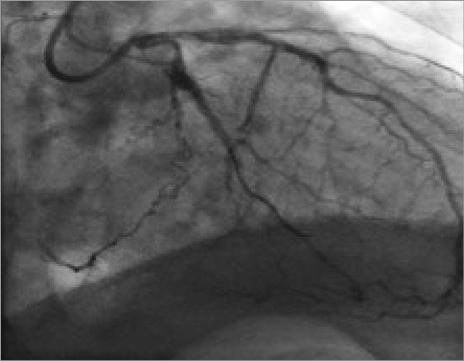
Figure 1. Subocclusive stenosis of the left main (LM).
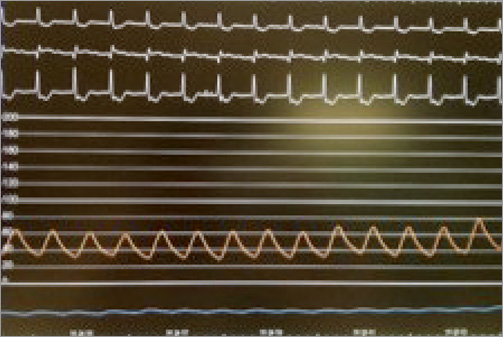
Figure 2. ST-T segment depression on electrocardiogram and drop of the arterial pressure.
Three days after the angioplasty, the patient underwent a CT angio scan, which showed: 1) the protrusion of the stent from the LM towards the aorta at approximately 6 mm (Figure 3); 2) an aortic annulus of 24 mm; 3) a maximum diameter of the sinus of Valsalva of 34 mm; 4) height of the left sinus of Valsalva of 14 mm; 5) the existence of atherosclerotic disease to both common iliac arteries, with a minimum diameter of 4.5 mm on both vessels, and the absence of circumferential calcifications.
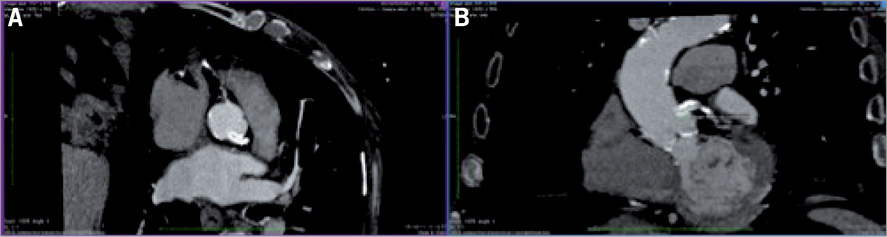
Figure 3. The protrusion of the stent from the LM towards the aorta.
How would I treat?
THE INVITED EXPERTS’ OPINION
In accordance with best practice, this case should be considered at an appropriate TAVI multidisciplinary meeting with attendant cardiac surgeons, interventional cardiologists and imaging specialists. From a valvular perspective this patient would appear to have symptomatic, severe aortic stenosis (assuming no resolution of his symptoms with LMS PCI). His high logistic EuroSCORE and age would suggest TAVI is appropriate. The iliofemoral vasculature precludes a transfemoral approach and we would therefore opt for either a transapical or a transaortic approach - the latter should only be undertaken in a centre with sufficient expertise given the coronary conundrum presented here. Given our experience of the device and the annular diameter of 24 mm we would choose a 26 mm Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA). Any further intervention should be delayed until at least 30 days from the LMS PCI to allow the patient to complete 30 days of dual antiplatelet therapy.
Concomitant coronary artery disease is frequently seen in the high-risk cohort of patients undergoing TAVI but the effects of treatment upon outcome are not known - though the ACTIVATION trial is currently enrolling to answer this question in patients without LMS disease1. The concern in this case is one of disruption of the LMS stent and coronary obstruction as a result of a TAVI. The former can be the result of displaced bulky calcification of the native leaflets, anatomical factors such as an annulus-ostia distance of <10-12 mm or a small sinus of Valsalva (SoV), an embolus or suboptimal prosthesis position.
Prevention being better than cure, we would secure the LMS/left anterior descending artery with a 0.014” guidewire, passing a compliant angioplasty balloon over the wire to check for smooth passage and ensuring that the guidewire is within the lumen of the stent rather than between struts and a precautionary balloon can be placed within the stent just prior to TAVI. The guide catheter should be withdrawn into the ascending aorta for the TAVI, leaving the guidewire as a marker. The transapical and transaortic Edwards SAPIEN XT systems use a shorter balloon for valvuloplasty and deployment and so would help avoid the aortic portion of the coronary stent. The valvuloplasty would give some indication of the likely impingement of the stent, and we would suggest a conservative 15 or 18 mm device to minimise that risk. The valve itself can be crimped such that there is minimal balloon on the coronary side of the device (i.e., distally for transapical and proximally for transaortic). The 26 mm device has a total height of 17.2 mm and so placement of the upper portion of the prosthesis below the left coronary ostium should be possible. The deployment should be done in a slow, controlled manner with reference to valve position and coronary patency assessed by angiography and echocardiography.
Preparation to prevent coronary obstruction, prompt recognition of hypotension and electrocardiographic changes, periprocedural transoesophageal echocardiography and a primed cardiopulmonary bypass facility in the laboratory are key to salvaging these difficult situations.
Conflict of interest statement
S. Redwood and M.Z. Khawaja have received research funding from Edwards Lifesciences and S. Redwood is a consultant to Edwards Lifesciences.
How would I treat?
THE INVITED EXPERTS’ OPINION
In this patient there is a risk of deforming/compressing the LM stent during valve implantation. This may induce early complications as well as compromise future access to the left coronary artery.
Preprocedural planning should include preventive measures (choice of appropriate approach and prosthesis) and bail-out strategies.
The transfemoral approach is not ideal because of small diameter iliac vessels. In addition, direct approaches may allow more controlled deployment. Further to the diagnostic vascular access (either radial or femoral) required to guide valve implantation, an additional vascular access is needed in order to take care of the LM stent (preferably a femoral approach).
Today three prostheses are available for clinical use, two self-expanding (CoreValve; Medtronic, Minneapolis, MN, USA, and Portico; St. Jude Medical, St. Paul, MN, USA), and one balloon-expanding (Edwards SAPIEN; Edwards Lifesciences, Irvine, CA, USA).
In our practice, for preference we would probably favour a 26 mm SAPIEN valve, which can be implanted below the LM ostium, with no impediment of future access to the coronaries. However, the main concern with a balloon-expandable valve is the risk of crushing/deforming the protruding struts of the LM stent during valve deployment. In this patient, the sinus of Valsalva dimension is 34 mm, and therefore the risk of touching the stent while deploying the valve is minimal. During balloon inflation, the LM stent should be protected with a coronary guidewire, or even intubated by a guiding catheter. Another option could be to leave a coronary balloon in the distal left main prior to valve deployment so that, in case the coronary stent is crushed, it will be easy to pull back the balloon and redilate the stent.
As an alternative to SAPIEN, a self-expanding valve could also be implanted, but with more challenges. With self-expanding valves, the coronary ostia will be covered by the valve struts, which could make a future coronary cannulation difficult, particularly if the coronary stent gets deformed by the valve. In addition, the protecting wire in the LM stent will be jailed. A St. Jude Portico valve has two theoretical advantages: it is repositionable and it is designed with larger stent cells. However, clinical experience with this valve is still limited, and only a 23 mm size is available at the moment. Therefore, this patient would not be a candidate due to his annular size (24 mm). A 29 mm Medtronic CoreValve would fit this patient. A lower implant is probably advisable to reduce the risk of covering the coronary stent, accepting a higher risk of AV block. Also in this case, a wire should be left in the coronary artery prior to deployment. After valve implantion, re-crossing with a coronary wire through the CoreValve struts may be needed to reorient the stent towards the valve struts, and to treat eventual stent compression.
In the future, another option for such cases might become available: the Direct Flow valve (Direct Flow Medical, Santa Rosa, CA, USA), which has just received CE mark approval. This device could be the best choice in this patient, since it is an inflatable repositionable device. It can be implanted below the coronary arteries, without a balloon, thus minimising the risk of damaging the LM stent.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular, Medtronic, St. Jude, and ValtechCardio, is a founder of 4Tech, and has received royalties from Edwards Lifesciences. A. Latib serves on a Medtronic advisory board and is a consultant for ValtechCardio and 4Tech.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The patient underwent a TAVI procedure with a CoreValve 29 mm prosthesis, through the right common femoral artery. The right common iliac artery was dilated with a 7×40 mm balloon, advanced on a 0.018” guidewire in the crossover position. After proceeding to position the Prostar® XL (Abbott Vascular, Santa Clara, CA, USA) haemostasis system, the SoloPath™ 14-18 Fr (Onset Medical Corporation, Irvine, CA, USA) expandable sheath was successfully inserted from the right femoral artery to allow for advancing the aortic prosthesis. We decided not to perform the preliminary valvuloplasty, to avoid the risk of completely crushing the stent struts protruding from the LM in the aorta. Finally, we proceeded with the direct implant of the CoreValve 29 mm prosthesis, with the constricted central area being 24 mm (Moving image 2). We therefore tried to utilise the remaining 10 mm of space in the sinus of Valsalva (approximately 5 mm on each side). The prosthesis implant procedure was successfully concluded (Moving image 3). The patient was discharged after six days in good clinical condition. At the one-month follow-up the patient showed a clear improvement of his functional capacity (NYHA Class I), and the echocardiography showed the normal functioning of the aortic bioprosthesis (valve area of 1.8 cm2/m2, mean transprosthetic gradient of 8 mmHg, absence of periprosthetic or intraprosthetic regurgitation). Finally, the angio CT scan revealed the correct position of the bioprosthesis and the patency of the stent in the LM, with partial “crushing” of the stent struts from the metallic elements of the prosthesis (Figure 4).
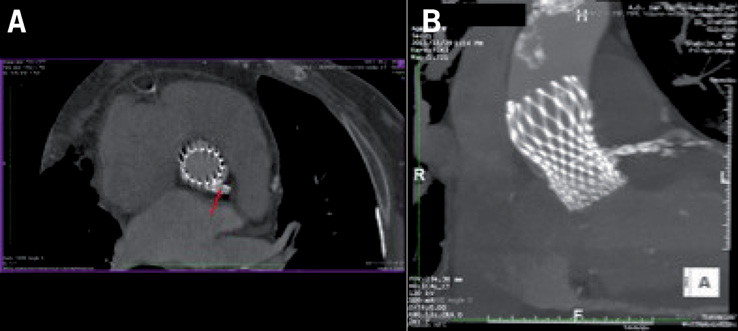
Figure 4. Partial “crushing” of the stent struts from the metallic elements of the prosthesis.
TAVI is recommended for inoperable patients with AVS and it is a reasonable alternative in high surgical risk patients2-4. A stent implanted on coronary ostia could be considered a worrisome element in patients with AVS undergoing TAVI, but this clinical case suggests the possibility of performing a TAVI procedure in patients with stents positioned in the ostium of the LM, protruding in the aorta, even without a preparatory valvuloplasty. The CoreValve implant in patients with coronary stents previously implanted in the coronary ostia may be performed keeping in mind the ratio between the width of the stent protrusion and the width of the sinus of Valsalva, which must be able to contain the valve and the protruding part of the stent. In fact, although minimal crushing of the most ostial part of the stent struts may be required, such a manoeuvre does not critically compromise the coronary flow, something that can occur from the lateral stent struts.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. PTCA of left main.
Moving image 2. The initial phase of TAVI release.
Moving image 3. Post-TAVI implantation angiography.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. PTCA of left main.
Moving image 2. The initial phase of TAVI release.
Moving image 3. Post-TAVI implantation angiography.

