CASE SUMMARY
BACKGROUND: A 74-year-old female with progressive fatigue and dyspnoea on exertion.
INVESTIGATION: Physical examination, laboratory tests, transapical echocardiography, transoesophageal echocardiography, coronary angiography, aortography, multislice computed tomography.
DIAGNOSIS: Severe aortic stenosis, left main obstruction following transcatheter aortic valve implantation.
TREATMENT: Transcatheter aortic valve implantation, emergent stenting.
KEYWORDS: aortic stenosis, transcatheter aortic valve implantation, left main obstruction, catheterisation, echocardiography
PRESENTATION OF THE CASE
A 74-year-old female with a history of long-standing rheumatic valvular heart disease presented to our centre with progressive fatigue and dyspnoea on exertion (New York Heart Association functional Class III). Transoesophageal echocardiographic (TEE) assessment revealed severe aortic stenosis (AS) (mean systolic gradient 48 mmHg; peak gradient 78 mmHg; aortic valve area 0.7 cm2; valve annulus 20 mm) and normal left ventricular function. A selective coronary angiogram revealed normal coronaries. Due to excessive surgical risk with conventional aortic valve replacement (AVR) (logistic EuroSCORE of 36%), the patient was scheduled for transapical aortic valve implantation (AVI). The procedure was performed under general anaesthesia, as previously described1. After aortography (Figure 1A, Moving image 1) and valvuloplasty, a 23 mm Edwards SAPIEN aortic valve (Edwards Lifesciences, Irvine, CA, USA) was implanted at the aortic annulus. Immediately after implantation, TEE illustrated the disappearance of diastolic coronary jet flow (Figure 1B, Moving image 2A) suggesting left main (LM) ostial occlusion, which was confirmed by simultaneous aortic angiography (Figure 1C, Moving image 2B), and the patient developed haemodynamic instability and cardiac ceramic arrest.
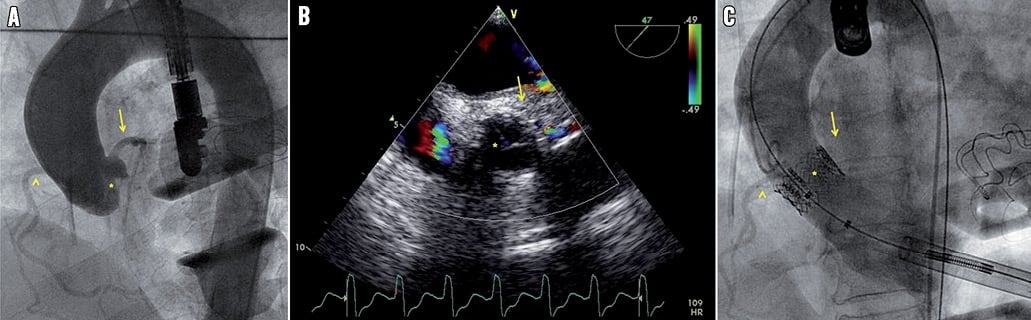
Figure 1. A) Aortic root angiogram before transapical aortic valve deployment depicting LM diastolic flow (arrow), RCA (arrow head) and a moderately to severely calcified native aortic valve (asterisk). B) Intraoperative TEE showing disappearance of LM jet flow (arrow) suggesting LM ostial obstruction. The asterisk refers to the native aortic valve. C) Aortography confirming the diagnosis of LM ostium occlusion (arrow). The arrow head refers to the RCA, and the asterisk refers to the prosthetic aortic valve.
How would I treat?
THE INVITED EXPERTS’ OPINION
Occlusion of the left main (LM) coronary ostium following transcatheter aortic valve implantation (TAVI) is a rare complication occurring in 0.4-4.1% of procedures and has been reported using both balloon-expandable and self-expandable valve prostheses2,3. This life-threatening event can occur immediately after TAVI (acute occlusion) or within the subsequent hours (delayed occlusion)2,4. Rapid deterioration of the haemodynamic status, electrocardiographic ischaemia and new wall motion abnormalities in the left coronary territory on echocardiography suggest this complication, and timely onset of haemodynamic support is crucial for its successful management.
The main cause of this complication relates to a patient’s anatomy: bulky leaflet tip calcifications or native leaflets obstructing the LM ostium, low take-off of the LM (ostium height to annulus <10-12 mm) or narrow aortic root at the level of the sinus of Valsalva which will prevent appropriate displacement of the native leaflets3,4.Three-dimensional imaging techniques, including transoesophageal echocardiography and multi-detector row computed tomography, are very accurate for evaluating the aortic root and valve anatomy prior to TAVI and for estimating the risk of this catastrophic complication4,5. Preventive measures include aortography during balloon valvuloplasty to evaluate the potential detrimental effect of valve leaflets or calcifications on coronary patency and additional arterial access or even preventive wire placement into the LM ostium3. Other procedure-related issues such as inappropriate high position of the sealing cuff of the prosthesis or (air) embolism have rarely been described as the cause of LM occlusion during TAVI4.
When confronted with acute LM occlusion, immediate circulatory support is indicated, and successful use of manual or automatic cardiopulmonary resuscitation, heart assist devices and intra-aortic balloon pump have been described in this clinical scenario2,3,6,7. Given the transapical approach in this patient, manual chest compressions should be started with the addition of extracorporeal membrane circulatory oxygenation (ECMO) since there is no coronary flow. If coronary obstruction is caused by calcium bulk or thrombus, a percutaneous approach is feasible: immediate LM access can be achieved by exchange of the pigtail catheter with a coronary guiding catheter, followed by coronary dilatation and stent implantation. If unsuccessful, bail-out coronary bypass grafting should be attempted.
In case of LM obstruction due to valve leaflet blocking and/or a too high aortic valve prosthesis position, immediate support with ECMO should be followed by urgent cardiac surgery, as percutaneous intervention is unlikely to be successful. This therapeutic option would be our choice for the case presented by Nazeri et al, showing LM ostium obstruction due to a too high aortic valve prosthesis position (Figure 1C) within an aortic root with low anatomic LM coronary ostium and small sinus of Valsalva (Figure 1A).
Conflict of interest statement
F. van der Kley is proctor for Edwards Lifesciences. V. Delgado received consulting fees from St. Jude Medical and Medtronic. The other authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
The most striking feature in the work-up of this case seems to be the relatively short distance between the virtual aortic annulus and the ostium of the left main stem particularly. One could have measured this distance by quantitative coronary angiography (QCA). However, multimodality imaging, and especially multislice computed tomography scanning, could prove to be the most reliable tool for this measurement. The 23 mm Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) valve has a height of 14.3 mm. The rule of thumb is to position the valve 50/50 relative to the virtual annulus, resulting in 7 to 8 mm of the skirt mounting into the aorta, potentially reaching up to the ostium of a shallow left main stem. Furthermore, the operator has to consider the lifting of the native valve leaflets along the aortic wall in the direction of the coronary ostia. Insufficient height from the annulus to the left main ostium can result in coronary occlusion by the displaced leaflets. If a distance <10 mm was confirmed I would have considered another transcatheter valve technique, either moving to a different access strategy (direct aortic access) or choosing a different device (Engager™ [Medtronic, Minneapolis, MN, USA], JenaValve [JenaValve GmbH, Munich, Germany], Symetis Acurate [Symetis SA, Ecublens, Switzerland]). If the Edwards balloon-expandable system had been selected I would have considered engaging the left coronary artery with a non-floppy 0.014” coronary guidewire before the actual valve implantation and specifically using the left main stem landmark to guide the actual valve implantation.
In this situation of acute left main stem occlusion my rescue strategy would be the following: 1) prepare and initiate cardiopulmonary bypass and in the meantime try to engage the left main stem with a stiffer coronary guidewire (e.g., an ASAHI MiracleBros 3; Abbott Vascular, Santa Clara, CA, USA); 2) if unsuccessful, attempt to grasp the Edwards device with two cardiac biopsy myotomes through the apical sheath and try to pull the Edwards frame in the direction of the left ventricle (although I have never tried this manoeuvre before!); 3) if unsuccessful, try to snare the Edwards THV coming from a femoral and radial approach and pull the device into the ascending aorta; 4) in the meantime, prepare the room for conversion to sternotomy and bypass the left anterior descending coronary artery (LAD) with a left internal mammary artery (LIMA).
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Cardiopulmonary resuscitation (CPR) started immediately, and the use of a mechanical assist device (intra-aortic balloon pump [IABP]) was considered simultaneously. At the same time, and with the help of haemodynamic support, obtained from chest compressions, the guiding catheter was placed at the presumed LM ostium (Figure 2A), and the guidewire was advanced through the prosthetic valve in the LM and the left anterior descending coronary artery (LAD) (Figure 2B). A 3.5 mm×12 mm XIENCE V stent (Abbott Vascular, Santa Clara, CA, USA) was successfully implanted after predilatation with two 1.5 mm×2.0 mm and 3.0 mm×12 mm balloon catheters at the occlusion site. After stent placement, cardiac function and haemodynamic status recovered rapidly (Figure 2C, Moving image 3). Post-procedure and pre-discharge TEE revealed normal left ventricular (no wall motion abnormality; ejection fraction 55%) and prosthetic valve function (peak gradient 12 mmHg; aortic valve area 2.2 cm2). The patient was discharged on post-procedural day five; her in-hospital stay had been uneventful. At the one-year follow-up, conventional coronary angiography (Figure 3A, Moving image 4) and 64-slice computed tomography (CT) (Figure 3B and Figure 3C) revealed the stent patency and normal left coronary arterial tree as well as the appropriate position and normal function of the prosthetic valve. At this time, she was symptom-free and had New York Heart Association functional Class I.
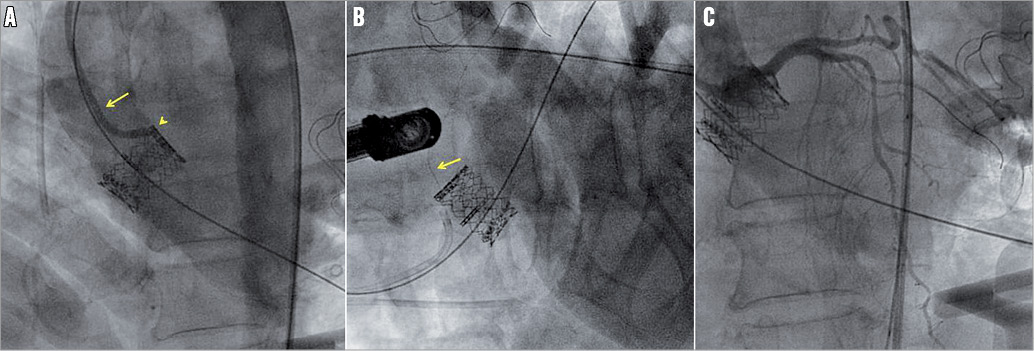
Figure 2. Management of the LM ostial occlusion, which occurred immediately after the prosthetic valve deployment. A) Guiding catheter (arrow) placed at the presumed LM ostium (arrow head). B) Guidewire (arrow) advanced through the prosthetic valve, in LM and LAD. C) Normal coronary flow restored after successful stent implantation at the occlusion site.

Figure 3. A) Control angiogram one year after the transapical AVI and stenting revealed a widely patent LM coronary artery at the site of stenting with excellent run-off (arrow), documenting the absence of the stent restenosis. (B) and (C) 64-slice CT scan at the patient’s one-year follow-up: volume-rendering reconstruction (B) and multi-planar reconstruction (C) demonstrate the implanted aortic valve and the stented LM coronary artery (arrows). Our case also confirms the importance of TEE as a useful diagnostic and monitoring tool during transcatheter AVI10. This technique, along with simultaneous angiographic control, enables continuous monitoring of the LM coronary flow and rapid diagnosis of the LM ostial obstruction during prosthetic valve deployment. In addition, intraoperative angiography provides perfect visualisation of the prosthetic valve and its relationship to the LM ostium throughout the valve deployment7.
Discussion
Surgical AVR remains the standard treatment of AS. Nevertheless, up to one third of patients with severe AS are estimated to have a very high or prohibitive surgical risk8. Recently, transcatheter AVI has emerged as an effective therapeutic alternative to conventional AVR for these patients9. The procedure, however, is associated with a risk of possibly fatal complications including LM coronary artery occlusion. Here, we have reported a case of LM trunk obstruction, which occurred acutely after transapical AVI; the case was diagnosed promptly with transoesophageal TTE and aortography and was successfully managed with stenting. The prosthetic valve function and position as well as stent patency were assessed at one year post procedure using follow-up selective angiography, aortography and 64-slice CT. This case reinforces the critical message that we must be vigilant to detect this uncommon complication of LM ostial occlusion, throughout and after transcatheter AVI. In this way, multimodality imaging, including TEE, invasive angiography and multislice CT (MSCT), plays a central role in the preoperative identification of high-risk patients, timely diagnosis of complications and follow-up of the implemented management5.
Transoesophageal echocardiography and MSCT provide accurate evaluation of the extent and location of aortic valve calcification in patients who are candidates for transcatheter AVI. This is crucial, because the presence of bulky calcifications may increase the risk of coronary ostium occlusion. Furthermore, MSCT is a comprehensive imaging technique for the measurement of the coronary ostia relative to the aortic valve annulus plane. A distance of less than 10 mm between the LM ostium and the aortic valve annulus plane increases the risk of coronary ostium occlusion5. In our case, the pre-procedural TEE and MSCT revealed a moderately to severely calcified valve with an appropriate LM ostium-to-aortic valve annulus distance (13 mm).
To the best of our knowledge, our case report is the first in which imaging has been employed for the long-term follow-up of a patient who has been managed by stenting the LM ostium after transapical AVI-related ostial occlusion. Multislice CT, as a non-invasive imaging modality, provides a highly accurate method for assessing the prosthetic valve function and position, and also for evaluating its spatial relationship with surrounding structures5. However, due to some limitations including impaired image quality and strut thickness, it has not been accepted as an alternative to invasive coronary angiography (the gold standard) for the evaluation of stent patency and for the detection of in-stent restenosis11. This was the reason why we used both MSCT and selective coronary angiography for evaluating the patient in her one-year follow-up.
As a conclusion, in order to achieve the best results and the lowest morbidity and mortality rates, transcatheter AVI should be performed in a fully equipped operating room and by a highly experienced team. This “circle of success”, undoubtedly, is not complete without the help of multimodality imaging.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Baseline aortic angiography shows normal diastolic filling of coronary arteries, including LM trunk, and moderately to severely calcified native aortic valve.
Moving image 2. (A) Intraoperative TEE showed no diastolic colour flow across the LM trunk suggesting LM ostial obstruction. (B) Simultaneous angiography confirmed the diagnosis.
Moving image 3. Post-stenting coronary angiography revealed restoration of normal coronary flow.
Moving image 4. Selective coronary angiography at one-year follow-up revealed normal left coronary arterial tree with excellent filling of the LM trunk, confirming the stent patency.
Supplementary data
To read the full content of this article, please download the PDF.

