CASE SUMMARY
BACKGROUND: A 77-year-old female with progressive dyspnoea.
INVESTIGATION: Physical examination, transthoracic echocardiography, transoesophageal echocardiography, computed tomography, coronary angiography.
DIAGNOSIS: Severe aortic stenosis of a bicuspid valve.
MANAGEMENT: Transfemoral transaortic valve implantation with a self-expanding prosthesis.
KEYWORDS: aortic stenosis, bicuspid valve, TAVI
PRESENTATION OF THE CASE
A 77-year-old female patient (height 146 cm, weight 52 kg) with progressive dyspnoea due to valvular aortic stenosis was referred to our hospital for TAVI. Transthoracic and transoesophageal echocardiography (TEE) showed a degenerated bicuspid aortic valve (Figure 1) with a peak velocity of 4.5 m/s (peak gradient 81 mmHg; mean gradient 52 mmHg, area of 0.5 cm2) in the absence of aortic and mitral regurgitation, a normal left ventricle function and absence of coronary artery disease. During discussion by the Heart Team, it was decided to perform TAVI because of antecedents of stroke with permanent hemiplegia. The logistic EuroSCORE was 14% (STS 5%). On computed tomography the common femoral artery right was 6 mm, left 7 mm in diameter. The dimensions of the annulus were: diameter minimum 18 mm, diameter maximum 26 mm, perimeter 68 mm, area 360 mm2. The Agatston score was 1,342 mg. Based on these measurements, a 26 mm self-expanding CoreValve® prosthesis (Medtronic, Minneapolis, MN, USA) was selected.
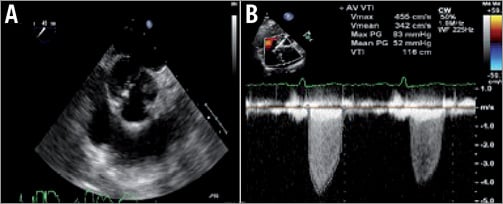
Figure 1. TEE showing bicuspid calcified aortic valve in short-axis plane (A). TTE showing continuous wave Doppler signal across the aortic valve (B).
TAVI was performed under general anaesthesia via the left femoral artery using an 18 Fr sheath as described before1. Retrograde crossing of the aortic valve with the use of a 0.035” Kimal® straight (Kimal, Uxbridge, UK) followed by a hydrophilic-coated Terumo Glidewire® (0.025”) (Terumo Corp., Tokyo, Japan) proved to be unsuccessful despite numerous attempts by all operators. For that reason, repeat analysis of the aortic valve with TEE was performed which revealed an eccentric jet with an angle of approximately 90 degrees with the axis of the ascending aorta, explaining the cause of the failure to cross the valve retrogradely (Figure 2). Attempts at retrograde crossing using echo guidance were continued but also proved to be unsuccessful. While the patient was under anaesthesia, consultation with all physicians present including the cardiothoracic surgeon was performed to decide the strategy that should be followed. Conversion to transaxillary TAVI was not possible due to the small size of both axillary arteries (<5 mm) and would not solve the problem of retrograde crossing2. The latter also holds true for a direct aortic access approach3. Conversion to transapical TAVI was deemed inappropriate because of the small size of the left ventricular cavity that could cause haemodynamic problems after insertion of a transapical sheath4.
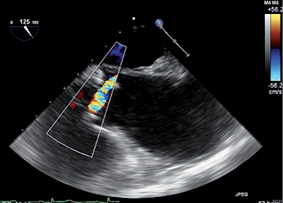
Figure 2. 2D TEE long-axis view with colour Doppler realised during the procedure showing an eccentric jet almost parallel to the aortic annulus.
How would I treat?
THE INVITED EXPERT’S OPINION
It is unusual not to be able to cross a stenosed aortic valve retrogradely. The usual equipment is an Amplatz Left 1 or 2 diagnostic catheter and a “straight” standard or hydrophilic wire. In the patient described, the difficulty to cross is secondary to the valve being bicuspid resulting in a highly eccentric Doppler jet. In this situation I would use a Judkins Right 4 diagnostic catheter and a hydrophilic wire. I would use 3D transoesophageal echocardiography to guide the position of the catheter, which, from Figure 2, should be in the left coronary sinus pointing anteriorly. I would be hopeful that in this position probing with a straight hydrophilic wire would result in successful valve crossing.
If, despite the above manoeuvre, retrograde crossing of the valve is impossible (as in this case), then antegrade crossing of the valve is the only solution. In my institution this could be achieved in one of two ways. The first would be to convert to the transapical approach. The authors rightly point out that a small left ventricular cavity can result in haemodynamic deterioration after insertion of the transapical sheath. Nonetheless, with an experienced team who work quickly I think this is an entirely reasonable option. We would not perform a balloon aortic valvuloplasty and would directly deploy the Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA) using the 22 Fr Ascendra Plus delivery system (Edwards Lifesciences). In the near future the next-generation SAPIEN S3 valve, using the 18 Fr Certitude delivery system (both Edwards Lifesciences), will result in much fewer haemodynamic problems using the transapical approach in small ventricles. Clearly a Heart Team approach with a close working relationship with surgical colleagues is required to facilitate this quick ad hoc change from the transfemoral to the transapical approach.
Should the transapical approach not be possible for some reason, then the second option would be direct left ventricular puncture with a thin needle and antegrade crossing of the valve5. The exchange crossing wire is then snared from the aortic side and externalised from the femoral sheath. Finally, an extra-stiff Amplatz wire is placed with the aid of a pigtail catheter. The needle can be withdrawn from the apex as soon as the wire has been externalised. Generally, no closure device is required with this approach, but the pericardial space can be monitored by the transoesophageal echocardiography during the procedure and surgical cover is required. Avoidance of trauma to the left anterior descending coronary artery during this manoeuvre can be facilitated by use of transthoracic echocardiography to identify the true apex and simultaneous coronary angiography as the left ventricular apex is punctured.
On balance, in my institution in this clinical situation we would treat by quickly converting to the transapical approach.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
In patients undergoing transvascular (predominantly transfemoral) transcatheter aortic valve implantation (TAVI), the retrograde crossing of the stenosed aortic valve is a condition sine qua non.
A variety of catheters can be used to cross the aortic valve retrogradely. To allow an easy orientation of the catheter tip and to direct the guidewire towards the aortic orifice, we preferentially use a 5 or 6 Fr Amplatz Left 1 in patients with a small and/or vertical aortic root and an Amplatz 2 in patients with a large and/or horizontal aortic arch. Furthermore, a multipurpose, Sones or right coronary catheter can be tried if the crossing manoeuvre with the Amplatz catheter is not successful.
Many operators tend to use a straight hydrophilic wire, which might be more atraumatic than a Teflon-coated guidewire but does not facilitate the crossing of the stenotic valve. Vice versa, an advantage of a PTFE-coated straight-tipped 0.035” guidewire seems to be its more stable position and easier control when facing the jet of the aortic stenosis.
An aortic root angiogram will help to appreciate the calcification of the valve and to determine the approximate site of valve opening. For crossing the aortic valve, the three-cusp view with perpendicular view of the aortic annulus might also be helpful. The tip of the catheter is positioned approximately 1 cm above the valve level and then slowly retracted while rotating clockwise and sequentially advancing the straight guidewire in systole and retrieving it in diastole. To decrease the risk of trauma to the left ventricle, a right anterior oblique (RAO) projection for the introduction of the catheter over the straight-tipped guidewire into the left ventricle can be used. Before advancing the preshaped stiff wire, the Amplatz catheter has to be exchanged over a 260 cm long exchange guidewire with J-tip for a pigtail catheter. The shape of the pigtail catheter facilitates the atraumatic introduction and safe placement of the preshaped stiff guidewire in the left ventricle. The shape of the stiff wire should take account of the size of the left ventricle with a larger curve for dilated left ventricles to protect the LV apex from perforation on the one hand and on the other hand to have enough support for aortic balloon valvuloplasty and valve deployment. The wire can be preshaped by the operator, e.g., with use of a syringe, or by the manufacturer (Safari wire with two curve sizes; Boston Scientific, Natick, MA, USA, or Confida wire with one curve size; Medtronic).
In this case, as described by Rodriguez-Olivares, several adverse circumstances hampered the retrograde passage of the stenosed aortic valve, caused a treatment strategy change, and finally led to a serious complication with fatal outcome for the patient. This 77-year-old female patient with bicuspid aortic stenosis had a small and horizontal ascending aorta, so that difficulties to cross the valve retrogradely could have been anticipated, if a transoesophageal echocardiogram had been performed before the procedure.
As there are unlimited combination possibilities, the operators could have used different catheter/wire combinations for the manoeuvre of crossing the aortic valve. They could have tried a PTFE-coated straight-tipped guidewire before considering alternative options. Although they did not show the aortic root angiogram, this has proven to be more helpful than echocardiography, especially in patients with complex anatomy. In addition, biplane fluoroscopy might have been helpful to anticipate the exact localisation of the aortic orifice.
It is worth discussing whether the patient really was inappropriate for a transapical approach due to the small size of the left ventricular cavity4. There would have been two transapical options: the “classical” transapical approach via minithoracotomy with antegrade valve deployment and the “percutaneous” transapical approach with puncture of the apex of the left ventricle, antegrade crossing of the aortic valve, and externalisation of the wire followed by retrograde deployment of the valve. This percutaneous transapical approach would have prevented transseptal puncture and manipulating with wires and catheters in the left ventricle to build an arteriovenous loop.
Compared to transapical TAVI, a small cavity size might also be a disadvantage when performing TAVI via a transseptal approach with the need to get a wire through the mitral valve and around many bends and curves to cross the aortic valve antegradely, then to exchange it against a long wire, and finally to externalise it with the use of a snare. Especially in older patients with frail tissue, this approach might also be traumatic. In addition, with a length of only 260 cm, the exchange wire might have transferred a lot of tension on the cardiac tissue, when the wire was externalised first and then a pigtail catheter was retrogradely advanced.
Finally, a more undersized balloon or even avoidance of BAV with direct implantation of the self-expanding CoreValve prosthesis might have been an option in this patient to prevent complications, as the cardiac tamponade which followed might also have derived from annular rupture6,7. Since it became obvious that BAV is not a prerequisite for the facilitation of valve deployment any more, several operators tend to use valvuloplasty balloons with a diameter close to the minimum diameter of the aortic annulus. Many centres would have used only an 18 mm valvuloplasty balloon.
If cardiac tamponade is a consequence of a tear in the left ventricle free wall caused by the stiff wire or catheters, this injury to the high-pressured left-sided circulation leads to death in more than half of the cases –even if the on-site Heart Team performs emergent cardiac surgery without delay7,8.
In conclusion, this difficult TAVI case emphasises the fact that even very experienced operators have to expect the unexpected – even after careful consideration of alternative treatment strategies in an elderly, frail TAVI patient– when facing unforeseen issues such as the impossibility to cross the aortic valve retrogradely. It ain’t over till it’s over.
Conflict of interest statement
J.M. Sinning has received research grants and speaker’s fees/honoraria from Medtronic and Edwards Lifesciences. E. Grube has received speaker’s fees/honoraria from and is a proctor physician for Medtronic.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
It was decided to cross the aortic valve antegradely via a transseptal approach and resume TAVI via the left femoral artery. A transseptal puncture was performed under TEE and followed by the advancement of a Terumo wire in the left ventricle using a Judkins Right (JR) catheter. After the antegrade crossing of the aortic valve with wire and JR, the Terumo wire was exchanged for a Medtronic (MDT) exchange wire (0.035”, 260 cm) which advanced in the ascending aorta (Figure 3). Via the left femoral artery, an Amplatz GooseNeck® Snare Kit (ev3 Endovascular, Inc., Plymouth, MN, USA) was used to snare the MDT exchange wire followed by exiting the wire via the sheath that was inserted in the left common femoral artery (Figure 4). Next, a 6 Fr pigtail was retrogradely advanced in the left ventricle and exchanged for an Amplatz Super Stiff™ (0.035”, 260 cm; Boston Scientific). Balloon valvuloplasty was then performed with a 22 mm balloon (Figure 5) and a 26 mm self-expanding prosthesis was then successfully implanted.
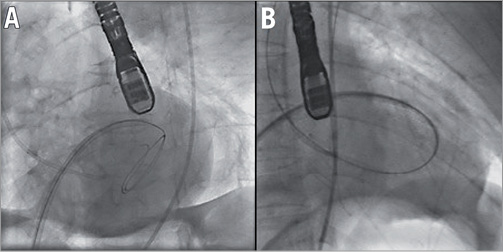
Figure 3. Transseptal puncture and positioning of hydrophilic wire and Judkins Right in the left ventricle (A). Anterograde crossing of the aortic valve and wire changed for exchange wire (B).
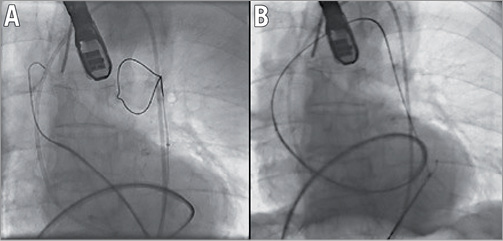
Figure 4. Amplatz GooseNeck® used to snare the exchange wire (A) and exit it via the left femoral artery (B).
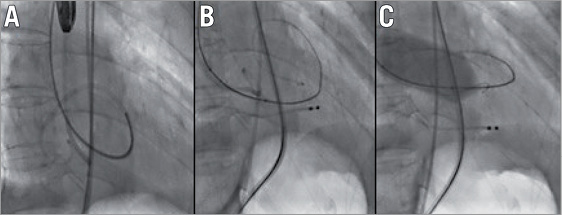
Figure 5. Retrograde insertion of pigtail via exchange wire (A), exchange for Amplatz Super Stiff wire (B), and valvuloplasty (C).
During the release of the valve, the patient suffered from hypotension. TEE immediately after implantation revealed a tamponade for which a drain was inserted. Because of persistent bleeding, thoracotomy was performed disclosing a perforation of the lateral ventricular wall that was repaired. The postoperative course was complicated by a lack of recovery of consciousness due to multiple watershed infarctions. The patient died one week after the index procedure.
This case illustrates the importance of a careful and in-depth preparation of a TAVI. The presence of an extreme eccentric jet was not recognised before TAVI. This should have informed us about the difficulty to cross the aortic valve retrogradely, and perhaps a transapical approach should have been considered despite concerns of the LV cavity. Perhaps we may add the following to the statement of Catherine Otto: “Aortic stenosis, listen to the patient, look at the valve and the jet!”9.
Conflict of interest statement
R. Rodríguez-Olivares receives a combined grant from EAPCI and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

