Case summary
Background: A 79-year-old female patient with past medical history of chronic myeloid leukaemia, presented to the outpatient cardiology clinic with shortness of breath (NYHA class 3) and fatigue.
Investigation: Echocardiography (ECG) and multislice computed tomography (MSCT).
Diagnosis: Severe calcified aortic valve stenosis by echocardiography. Logistic EuroSCORE 10.3. Porcelain aorta by multislice computed tomography (MSCT).
Treatment: Transcatheter aortic valve implantation (TAVI) with an 18 Fr Medtronic-CoreValve system (Medtronic Inc., Minneapolis, MN, USA) using a right transfemoral arterial approach.
Keywords: TAVI, aortic valve
How should I treat?
Presentation of the case
A 79-year-old female patient with past medical history of chronic myeloid leukaemia, presented to the outpatient cardiology clinic with shortness of breath (NYHA class 3) and fatigue. ECG showed a sinus rhythm with a narrow QRS complex and left ventricular hypertrophy. Echocardiography revealed a preserved systolic left ventricular function and a calcified severely stenotic aortic valve with a peak velocity of 4.5 m/sec and a calculated aortic valve area of 0.8 cm2 (Figure 1).
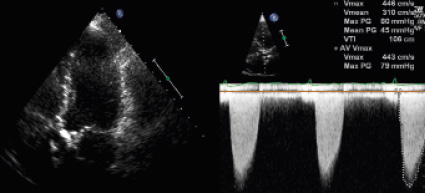
Figure 1. Transthoracic echocardiography: calcified aortic valve with high velocity across the aortic valve by Continuous Wave Doppler.
There was mild aortic and mitral valve regurgitation. Multislice computed tomography (MSCT) demonstrated a porcelain aorta with a wide ascending segment (diameter 4.5 cm) (Figure 2), bilateral moderate atherosclerotic carotid artery disease and patent 6 mm calibre common femoral arteries.
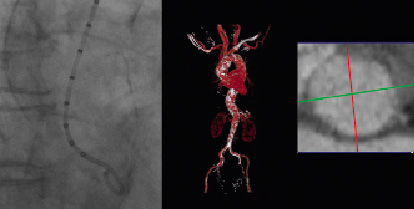
Figure 2. Angiographic (left) and MSCT confirmation of the porcelain aorta and aortic annulus measuring 21 x 23 mm.
The aortic annulus by MSCT was 21x23 mm. The biochemistry panel was unremarkable. Estimated glomerular filtration rate was 68 mL/min. The calculated logistic EuroSCORE was 10. At the heart team discussion the cardiothoracic surgeon and the interventional cardiologist considered the patient a good candidate for TAVI mainly because of frailty and the porcelain aorta.
The TAVI procedure was executed according to local expertise as previously described1. Right femoral arterial and venous access was obtained with ultrasound guidance. A temporary pacemaker was positioned in the right ventricular apex and a 6 Fr marker pigtail was introduced through the left radial artery into the nadir of the non-coronary aortic cusp. After upgrading the femoral arterial sheath to an 18 Fr system and crossing the calcified aortic valve, balloon valvuloplasty was performed with a 22 mmx4 cm Nucleus balloon (NuMED, Canada Inc., Cornwall, ON, Canada) over an 0.035”x300 cm extra stiff back-up Meier guidewire (Boston Scientific Inc., Miami, FL, USA) (Figure 3, Movie 1) generating severe aortic regurgitation.
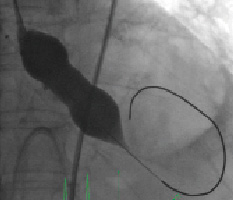
Figure 3. Aortic balloon valvuloplasty with a Nucleus 22 mm x 4 cm balloon.
Subsequently, we failed to cross the dilated aortic valve with a 26 mm Medtronic CoreValve system. Considerable resistance was encountered while attempting to advance the device through the aortic valve into the left ventricle outflow tract (Figure 4, Movie 2).
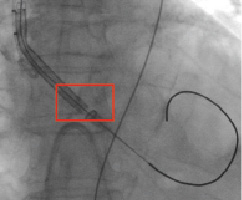
Figure 4. Medtronic CoreValve system cannot cross the heavily calcified aortic valve (red box).
Despite considerable push on the delivery catheter on various occasions over the Back-up Meier guidewire (Boston Scientific Inc., Natick, MA, USA), we failed to cross the aortic valve.
How would I treat?
The Invited Experts’ opinion
Most probably, the Back-up Meier guidewire (Boston Scientific Inc., Natick, MA, USA), which is an even more stiff wire compared to the most frequently used Amplatz Super Stiff™ wire (Boston Scientific Inc.), is entrapped between the aortic valve leaflets. Moreover, it might also be that the stenotic aortic valve is insufficiently predilated. Therefore, I should suggest the following manoeuvres:
“pull and push”
Gently pull on the Back-up Meier guidewire and try to bring it somewhat more central into the aortic valve, before pushing on the CoreValve device (Medtronic Inc., Minneapolis, MN, USA). By pulling on the wire, wire entrapment between the valve leaflets can sometimes be resolved.
“re-dilate”
When the CoreValve device still can not be advanced through the aortic valve into the left ventricle outflow tract, remove the device and predilate again the native valve and optimise the native valve opening using the Nucleus balloon (22 mm x 4 cm) (NuMED, Canada Inc., Cornwall, ON, Canada) during rapid pacing. Then again reintroduce the CoreValve device and try to cross again.
“recross + buddy wire”
When the native valve still can not be crossed, again remove the CoreValve device, but leave the Back-up Meier guidewire in place. By using for ex a 5 Fr left Amplatz I catheter and a straight Terumo wire (Terumo Corp., Tokyo, Japan), again cross the native aortic valve and insert the left Amplatz catheter into the left ventricle. Introduce a pre-shaped Amplatz superstiff wire through the Amplatz catheter into the left ventricle. Re-introduce the CoreValve device over the newly placed Amplatz stiff wire into the ventricle. The initially placed Back-up Meier wire remains in place and functions as a “buddy wire”. If the native valve can be crossed, remove the buddy wire before implanting the CoreValve device.
“snare technique”
When the native aortic valve again can not be crossed, the use of a snare inserted via the femoral artery can change the entrance position of the CoreValve device into the native aortic valve.
Reference
How would I treat?
The Invited Experts’ opinion
The principle challenge described herein by the authors, namely difficulty crossing the stenotic aortic valve with the 18 Fr Medtronic CoreValve delivery catheter, occurs rarely. A PubMed literature search did not disclose a similar case report with the Medtronic CoreValve device. Furthermore, in our last 300 procedures using the CoreValve device, we haven’t encountered a similar problem.
From the case description, it appears that the delivery catheter is encountering a mechanical obstruction at the level of the native aortic valve. The delivery catheter may simply be entrapped by a calcific nodule or within an aortic valve commissure. Although less likely, an aortic dissection needs to be ruled out. In any case, transesophageal echocardiography and fluoroscopy should be performed to investigate the cause.
The first reflex may be to “push harder and harder” until the delivery catheter crosses the stenotic aortic valve. We strongly advise against using such an approach. On the other hand, the use of “gentle force” while advancing the delivery catheter with a clockwise and counter-clockwise rotation can be attempted.
The role of pre-implant balloon aortic valvuloplasty, in addition to improving the “seating space” for valve deployment, is to sufficiently enlarge the valvar orifice and facilitate retrograde crossing of the stenotic aortic valve with the delivery catheter. In the present case, the aortic annulus measured 21x23 mm by MSCT and pre-implant BAV was performed with a 22 mmx4 cm Nucleus balloon (NuMED Canada Inc. Cornwall, ON, Canada). Based on the size of the aortic annulus and history of porcelain aorta, we agree with the 22 mm diameter balloon for aortic valvuloplasty. Inspecting Figure 3, however, it is unclear if maximal balloon expansion was obtained or if rapid pacing was instituted during valvuloplasty. Accordingly, repeat BAV during rapid ventricular pacing would be recommended to adequately enlarge the valvar orifice using the same Nucleus balloon (dog-bone balloon) or alternatively a 6-cm long straight balloon such as the Z-MED or Tyshak (straight balloon) (NuMED Canada Inc., Cornwall, ON, Canada).
A calcific nodule or fused commissure located along the path of the Back-up Meier guidewire (Boston Scientific Inc., Miami, FL, USA) may potentially be creating a mechanical obstruction to the passage of the delivery catheter. In this case, withdrawing the Back-up Meier guidewire from the left ventricle and re-crossing the valve in the usual manner, may lead to a different positioning of the wire across the aortic valve and thereby sidestep the mechanical obstruction. Even after re-crossing the valve, “wire bias” may direct the guidewire into a similar position as in the first place. Thus, it is important to appreciate if the wire has indeed changed position with either fluoroscopy and/or transesophageal echocardiography. If this manoeuvre is unsuccessful, consideration should be given to using a “buddy wire” technique.
The “buddy wire” or “buddy balloon” technique is occasionally used during percutaneous coronary interventions to facilitate crossing of tortuous or calcified segments1. The principle behind the technique is to provide extra support and deflect the stent delivery system away from the calcified plaque or obstruction. In the case at hand, a “buddy wire”, “buddy catheter” or “buddy balloon” can conceivably facilitate retrograde crossing of the delivery catheter through the aortic valve. The addition of a second extra stiff wire through the contralateral femoral artery and into the left ventricle may favourably remodel the anatomy, deflect the delivery catheter, or provide a second railway that avoids the initial wire bias that caused the obstruction. Alternatively, a pigtail catheter or aortic valvuloplasty balloon may be used to deflect the transcatheter aortic valve delivery system. Having said that, Sheiban et al recently reported a case in which a buddy wire and balloon were used as a “shoehorn” to enable delivery of a transcatheter aortic valve through a troublesome stenotic aortic valve2.
Also to consider is the delivery catheter. It should be examined ex vivo to ensure the valve is correctly loaded (specifically, that the delivery sheath and delivery cone are in flush contact to avoid entrapment of the delivery sheath with aortic root structures).
How did I treat?
Actual treatment and management of the case
The case was part of a live presentation at the Rotterdam TAVI training course in December 2009. Several possible solutions were considered. We first performed additional balloon valvuloplasty with a 23 mmx6 cm Z-MED-II-X balloon (NuMED Canada Inc. Cornwall, ON, Canada) (Figure 5, Movie 3).
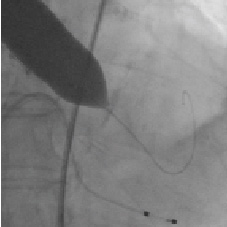
Figure 5. Second balloon valvuloplasty with a 23 mm x 6 cm Z-MED-II-X balloon.
This did not result in a successful crossing of the Medtronic-CoreValve system through the native aortic valve. We then tried to snare the nose cone of the delivery system with an Amplatz Gooseneck. Also changing the angle of approach by making an extra bend on the super-stiff part of the Back-up Meier that was located in the ascending aorta did not allow the crossing of the prosthesis. This was followed by the exchange of the Back-up Meier for a 0.035”Amplatz Super Stiff (Boston Scientific Inc., Natick, MA, USA) guidewire. Crossing of the aortic valve remained impossible. The aortic valve was then crossed with a second Back-up Meier guidewire serving as a modified buddy wire. Again we could not advance the Medtronic CoreValve system into the left ventricle. We then decided to exchange the buddy Back-up Meier for a pigtail catheter to fill up the commissure between the non- and right aortic cusp in addition to a bend on the Back-up Meier wire (in the rigid segment just proximal to the floppy part) so as to direct the device away from the aortic wall more centrally into the aortic valve orifice (Figures 6a + 6b + Movie 4).
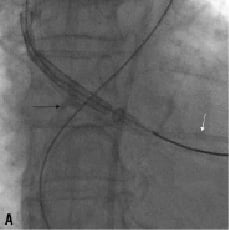
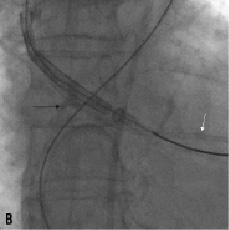
Figure 6. A. The delivery system is redirected away from the aortic wall crossing the aortic valve (black arrow). Also note the pigtail catheter (white arrow) in the left ventricle. B. Artificial bending of the Back-up Meier wire in its rigid segment (arrow)
This time the device was smoothly delivered into the left ventricular outflow tract (Movie 5).The bioprosthesis was then positioned and deployed across the valve with a good final angiographic result confirmed by transoesophageal echocardiography (Figure 7, Movie 6+7).
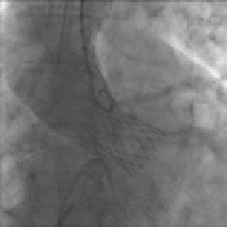
Figure 7. Angiogram Medtronic CoreValve System in situ with no residual aortic regurgitation.
Discussion
For the first time in 125 implantations at the Thoraxcenter we encountered considerable difficulties in delivering the Medtronic Corevalve bioprosthesis into the native aortic valve despite a successful prior balloon valvuloplasty. The aetiology of the failure of crossing is unknown. A residual piece of calcium may have impeded crossing, or the guidewire may have been trapped in an irregular structure of the aortic annulus after the balloon valvuloplasty. Another reason may be an unfavourable angle of approach as a result of the outward push by the incoming crimped bioprosthesis and insufficient support/trackability of the extra stiff wire. Several tips and tricks to overcome this hurdle are presented here. The concise interpretation and appropriate skill sets to overcome procedure related obstacles are fundamental for success in this fairly new domain and will help make this technology push its limits to a broader spectrum of patients with symptomatic aortic valve stenosis.
Online data supplement
Movie 1. Cine-angiography of balloon valvuloplasty with a 22 mm x 4 cm Nucleus balloon.
Movie 2. Cine-angiography demonstrating failure to cross the aortic valve with the CoreValve System.
Movie 3. Cine-angiography of balloon valvuloplasty with a 23 mmx6 cm Z-MED-II-X balloon.
Movie 4. Illustration of bending the Back-up Meier wire (in the rigid segment just proximal to the floppy part).
Movie 5. Cine-angiography of the CoreValve system crossing the aortic valve.
Movie 6. Cine-angiography after CoreValve deployment.
Movie 7. Transesophageal exam with the CoreValve in place.

