CASE SUMMARY
BACKGROUND: A 62-year-old man was admitted for severe heart failure. Echocardiography revealed an aortic stenosis and a poor left ventricular ejection fraction (LVEF) with an intraventricular blood clot. The situation was transiently stabilised with dobutamine and furosemide but, after a few days, he developed heparin-induced thrombocytopaenia. Other comorbidities included renal failure and hypertension. For all these reasons, conventional surgical aortic valve replacement was contraindicated.
INVESTIGATION: Echocardiographic mean transvalvular gradient was measured at 30 mmHg and aortic valve area at 0.4 cm2. LVEF was estimated at 25%. Multislice computed tomography (MSCT) confirmed the feasibility of the transfemoral approach. The preprocedural diameter of the native aortic root was 25 mm. The patient had normal coronary arteries.
DIAGNOSIS: Severe calcified aortic stenosis associated with left ventricular failure not allowing cardiac surgery. The logistic EuroSCORE was calculated at 35% and the STS score at 28%.
MANAGEMENT: We decided on transcatheter aortic valve implantation (TAVI) via a femoral route with a 29 mm CoreValve® prosthesis (Medtronic Inc., Minneapolis, MN, USA).
KEYWORDS: aortic valve stenosis, cardiac arrest, cardiopulmonary resuscitation, mechanical chest compression, transcatheter aortic valve implantation (TAVI)
PRESENTATION OF THE CASE
A 62-year-old man was admitted to our intensive coronary care unit as an emergency for symptomatic heart failure (lung congestion, pleural effusion, and lower extremity oedema). He knew he had a heart murmur and presented dyspnoea (New York Heart Association [NYHA] Class III symptoms) for a few months but had never been referred for echocardiography. Transthoracic echocardiography revealed a very poor left ventricular ejection fraction (LVEF <25%) with an intraventricular blood clot and a severely calcified trileaflet aortic stenosis. The maximum transvalvular gradient was measured at 80 mmHg and the mean gradient at 30 mmHg, but the aortic valve area was under 0.4 cm2. The patient was anticoagulated with continuous infusion of heparin, and the situation was stabilised by a few days of infusion of inotrope (dobutamine) and furosemide. Coronary angiography was performed and showed normal coronary arteries. Other comorbidities included renal failure and hypertension. The logistic EuroSCORE was calculated at 35% and the STS score at 28%. Then the patient was discussed by the Heart Team and was accepted for surgical aortic valve replacement on condition of a good dobutamine-induced recovery of systolic function and disappearance of the intraventricular blood clot.
However, the evolution was not good, and eight days later congestive signs reappeared with thrombopaenia. This was heparin-induced and the patient received Orgaran® (danaparoid). Moreover, the clot was not visualised again in the left ventricle. At this time, the Heart Team decided on a TAVI procedure under local anaesthesia. Multislice computed tomography (MSCT) confirmed the feasibility of the transfemoral approach.
After the usual beginning of the transfemoral CoreValve® implantation procedure, a Super Stiff™ guidewire (Boston Scientific, Natick, MA, USA) was inserted in the left ventricle for balloon angioplasty. However, before balloon inflation, the patient presented worsening hypotension, pulseless electrical activity and was unresponsive. The guidewire was retrieved, the procedure disrupted and a cardiopulmonary resuscitation was performed with chest compressions and rescue breaths in a ratio of 30:2. Adrenaline was infused at 3 mg every three minutes and the patient was intubated for mechanical ventilation.
However, resuscitation was not successful, and after 10 minutes of well-conducted reanimation1 we understood that return of spontaneous circulation (ROSC) was not probable because of left ventricle failure and valvular disease.
How would I treat?
THE INVITED EXPERTS’ OPINION
A patient with severely impaired left ventricular function requires special consideration and preparation prior to transfemoral TAVI. It is reasonable to perform such cases under general anaesthesia with intubation to avoid emergency conversion. Complete monitoring with a central venous catheter, the use of a Swan-Ganz catheter, insertion of a transoesophageal probe and arterial pressure monitoring are routine at our centre for patients with an LVEF <20%. As for all TAVI procedures, a heart-lung machine is ready for use and, in addition to the arterial access in both femoral arteries (18 Fr sheath in one groin for the valve implantation, and 5 or 6 Fr sheath for the pigtail catheter in the other groin), a venous guidewire is placed in one femoral vein2. If cardiac deterioration occurs, haemodynamic measurements and transoesophageal echocardiography may help to identify and address causes, such as pericardial tamponade or coronary obstruction, which require specific treatment. If no specific reason for the deterioration can be identified, the cannulas for the heart-lung machine can be inserted into the femoral artery and vein early after the start of resuscitation. It may happen that the patient recovers during this manoeuvre. If not, cardiopulmonary bypass can be installed quickly within 5-10 minutes. In the described case from Leroux and co-workers, severe hypotension occurred before valvuloplasty and valve implantation. We would then conduct the complete TAVI procedure under cardiopulmonary bypass and stable conditions. Usually, the patient can then be weaned from cardiopulmonary bypass within 30 minutes, as the left ventricular afterload is significantly decreased after valve implantation supporting recovery of the ventricle.
Out of 943 TAVI cases at our institution from June 2007 to January 2013, 30 patients (12 with severely impaired left ventricular function) exhibited severe hypotension requiring resuscitation before or after valve implantation without any additional complication such as tamponade or coronary obstruction, and five were put on a heart-lung machine. All of these could be weaned successfully from the heart-lung machine and survived to hospital discharge.
In conclusion, a differentiated treatment of patients at high risk for cardiac decompensation during a TAVI procedure is paramount. Prophylactic extracorporeal support or the availability of a heart-lung machine within minutes is highly effective in restoring circulation and gas exchange in patients with cardiocirculatory failure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
“If anything goes wrong, fix it” (Peter Safar, 12 April 1924 - 2 August 2003)
Assuming cardiac tamponade has been excluded (procedure-guided TOE or focused TTE), we would proceed with advanced mechanical circulatory support (MCS). This aims to maintain blood flow and oxygen delivery to dependent organ systems, thereby preventing multiple organ dysfunction and subsequent death, whilst the underlying cause for critical deterioration is reversed. A minimal flow rate of 70 mL/kg body weight per minute (cardiac index >2.5 L/m²) is generally required to provide adequate organ perfusion, equalling the sum of MCS output and the remaining intrinsic cardiac output. A range of potential methods is available; however, where active cardiopulmonary resuscitation is underway in the catheterisation laboratory, rapid percutaneous institution of venoarterial extracorporeal membrane oxygenation (ECMO) is a valuable treatment option3,4. As this patient is already instrumented in preparation for TAVI, cardiopulmonary bypass can be rapidly instituted using standard techniques4. Compact, portable devices with rapid initiation and easy manipulation are key in this setting5-7; however, the best device is the one with which the team are most familiar. The aim is to provide respiratory and/or circulatory support allowing for either surgical aortic valve replacement or TAVI. Parental direct thrombin inhibitors may be used for anticoagulation in the presence of heparin-induced thrombocytopaenia on ECMO7,8 resulting in a dose-dependent increase in activated partial thromboplastin time allowing for simple monitoring9. Additional care must be taken to avoid downward displacement of the TAVI, and/or thrombosis of the implanted valve during extracorporeal support due to absence of left ventricular ejection/aortic valve opening.
Conflict of interest statement
S. Price has an educational contract with Medtronic and is an advisory board member for MitraClip, Abbott. P. Vranckx has no conflicts of interest to declare in relation to this paper.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Resuscitation was not successful and, after 10 minutes of well-conducted reanimation, we had the choice to manage the patient with extracorporeal life support (ECLS) or to try to implant the valve during resuscitation. For this second option, the questions were: is this procedure realistic and can it really increase the possibilities of ROSC?
So, we decided to use the LUCAS® (Lund University Cardiopulmonary Assist System; Medtronic) device to perform TAVI during automated cardiac massage. LUCAS® provides active compression-decompression cardiopulmonary resuscitation. Using this device, the guidewire was inserted again through the aortic valve. LUCAS® was paused for a few seconds for balloon angioplasty with a 25 mm NuCLEUS™ balloon (Numed Inc., Hopkinton, NY, USA) and again for a few seconds for the 29 mm CoreValve® deployment (Figure 1). Three minutes after valve implantation and after a total adrenaline dose of 25 mg, stable haemodynamic conditions were rapidly obtained. LUCAS® was stopped after 20 minutes of mechanically assisted resuscitation. Total duration of the cardiac arrest had been 30 minutes.
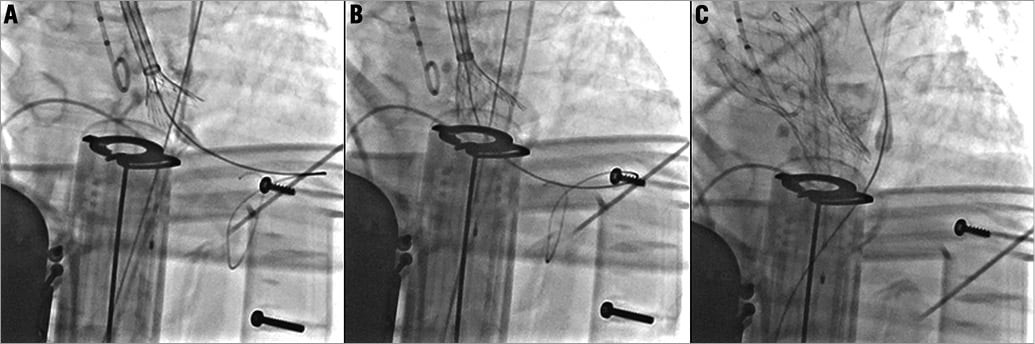
Figure 1. CoreValve implantation during cardiopulmonary resuscitation with the LUCAS device.
The patient was then admitted to the intensive coronary care unit and underwent 24 hours of therapeutic hypothermia at 34° as recommended by international guidelines1. The transthoracic echocardiogram revealed a good functioning of the prosthesis. Seventy-two hours later, after interruption of sedation, the patient recovered consciousness but was kept under respiratory assistance because of a transient alveolar haemorrhage (Figure 2).
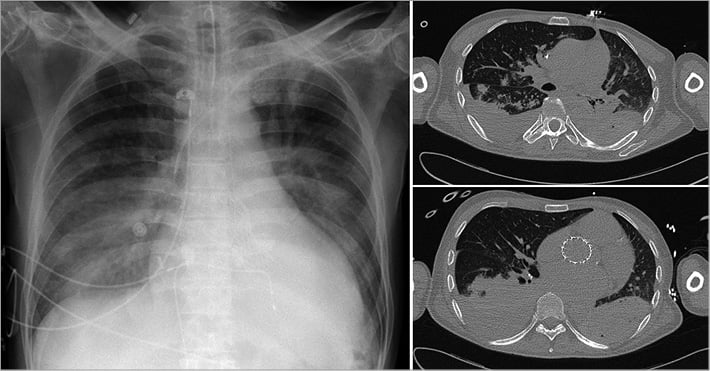
Figure 2. CT scan showing alveolar haemorrhage after 30 minutes of LUCAS-assisted resuscitation.
Unfortunately, the patient died at day five as a result of an acute intracerebral haemorrhage.
Discussion
Resuscitation of cardiac arrest in patients with aortic stenosis or regurgitation generally gives bad results. The main cause is the inefficiency of the external cardiac massage due to the presence of aortic valve disease. Transcatheter aortic valve implantation (TAVI) has emerged as a promising alternative technique to treat aortic stenosis in patients at high surgical risk10,11. Patients with poor left ventricular ejection fraction are good candidates for TAVI. Thus, cardiac arrest during a TAVI procedure is rare but usually with dramatic consequences. Indeed, cardiac resuscitation needs interruption of the procedure and does not allow treatment of the cause of failure.
Moreover, mechanical devices for automated cardiac compression are being implemented in routine clinical practice in spite of limited evidence to support their use from clinical outcome studies12. Intuitively, these devices have advantages such as providing consistent compression rate allowing defibrillation during ongoing chest compressions and freeing the hands of the rescue team. In 2002 in Sweden, a device named LUCAS® was proposed as an easy-to-use and quick-to-mount device for automated external cardiac massage, even during transport13 (Figure 3). The LUCAS® is a pneumatic mechanical pump which performs active compression as well as decompression with a pneumatic force of 500N on the thoracic wall at a frequency of 100 cycles/min. Another device currently available for mechanical chest wall compression is the AutoPulse (ZOLL Medical Corporation, Chelmsford, MA, USA). It is constructed around a backboard that contains a motor to retract a load-distributing band that is applied across the entire anterior chest. It is programmed to provide a constant 20% reduction in the anterior-posterior dimension of the patient’s chest during the compression phase. However, the backboard of the AutoPulse is not translucent enough for X-radiation actually to be used in the catheter laboratory. The LUCAS® device has been experimentally evaluated in cardiac arrest studies, where it has been shown to increase cortical cerebral blood flow and cardiac output13,14. In the first small human studies, mechanical chest compressions with the LUCAS® device for out-of-hospital cardiac arrest did not improve clinical outcome15,16. Moreover, very recently, Ong et al17 showed, in a larger cohort, a benefit on neurological outcome after cardiac arrest treated with the AutoPulse. However, because the device is translucent for X-radiation, its interest has been successfully tested in the angioplasty setting18-21. However, its usefulness for cardiac arrest during TAVI has never been demonstrated.
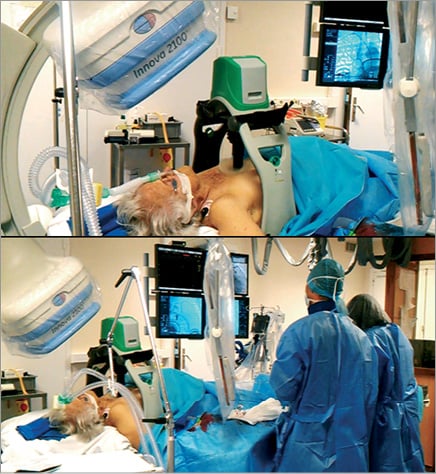
Figure 3. LUCAS device allows mechanical chest compressions even during interventional cardiology procedures.
Some case reports have been published showing the feasibility of different techniques of rescue for cardiac arrest during TAVI. Crimi et al22 reported that internal cardiac massage during a transapical Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) implantation complicated by an occlusion of the left main artery was sufficient to support the patient until the left main occlusion recovered. In a similar case of left main trunk occlusion with cardiac arrest after transfemoral implantation, Kapadia et al23 used a TandemHeart® LV assist device (CardiacAssist, Inc., Pittsburgh, PA, USA) to stabilise the patient and then successfully performed a left main artery angioplasty. We have to note that in this case this solution had been planned during the preparatory phase for potential catastrophic complications. In such cases of coronary artery origin of heart failure, we can also imagine using the Impella® assist device (Abiomed, Inc., Danvers, MA, USA) which can be rapidly implanted. However, in our case the aortic valve itself was involved in the mechanism of the cardiac arrest and the cardiac support solution had to keep the femoral and aortic route as well as the aortic valve free. Of course surgical rescue can be planned in this type of emergency situation, but it does not seem to be an optimal solution for these surgically high-risk patients: if a planned surgery is high risk then emergent surgery tends to be even higher risk.
Moreover, we have to note that our patient presented a post-resuscitation transient alveolar haemorrhage, which delayed extubation. This lung injury was similar to a post-traumatic pulmonary contusion and was attributed to the sustained cardiac massage in a patient treated with danaparoid. Device-related injuries have of course been described with the two devices available. In 2007, Rubertsson et al14 described complications such as rib and sternal fractures, but these are thought to be no more common than those occurring with manual chest compressions. Of note, a case of a device-induced pneumothorax has been published24, and a LUCAS®-resuscitated woman died after presenting an abdominal bleeding due to a laceration of the liver25. Indeed, Blomberg et al26 showed recently that incorrect application or sliding of the LUCAS® during ongoing compressions generated inadequate compressions and could erase the benefit of mechanical chest compressions. A first series of 11 autopsies after unsuccessful LUCAS-CPR showed all these types of injury19 but, in a paper by Smekal et al27, autopsies revealed no difference in injuries on 85 unsuccessfully resuscitated patients who received either manual chest compressions or LUCAS®. In the two arms they observed some cases of retrosternal, mediastinal or epicardial bleeding: one patient in the LUCAS® group had a small rift in the liver and one patient in the manual group had a rift in the spleen.
In our report, alveolar bleeding was the only injury identified and would probably have been transient, but these data support the idea of an educational programme before using LUCAS® in the catheter laboratory.
In summary, our case suggests that the x-ray translucent LUCAS® device can now be included among tools to manage cardiac arrest during transcatheter aortic valve implantation. Of course, a short but obligatory team educational programme is essential for successful rescue.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. CoreValve® positioning during LUCAS cardiopulmonary resuscitation.
Moving image 2. Ultimate aortography after valve deployment.
Supplementary data
To read the full content of this article, please download the PDF.

