- accelerated idioventricular rhythm
- reperfusion
- acute myocardial infarction
- haemodynamics
Abstract
Aims: Accelerated idioventricular rhythm (AIVR) is very frequently observed in primary percutaneous coronary intervention (PCI), however knowledge of the haemodynamic effects is lacking.
Methods and results: We studied an ST-segment elevation myocardial infarction cohort of 128 consecutive patients (aged 62±11years) in whom AIVR occurred following reperfusion during primary PCI. Mean systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate were determined during periods of AIVR and sinus rhythm. We grouped patients according to the infarct-related artery and the site of the coronary occlusion. AIVR caused an immediate reduction in SBP (130±27 vs. 98±22mmHg, p<0.001) and DBP (80±19 vs. 69±16mmHg, p<0.001) and a small increase in heart rate (78±12 vs. 83±11bpm, p<0.001) as compared to sinus rhythm, irrespective of infarct-related artery. Both absolute as well as relative reduction in SBP were more pronounced in distal than proximal left coronary artery (LCA) occlusions (36±16 vs. 27±12mmHg, p<0.01, respectively 25±9 vs. 20±8%, p<0.05). These haemodynamic differences between proximal and distal occlusion sites were not observed in the right coronary artery.
Conclusions: AIVR following reperfusion is associated with marked reduction in both SBP and DBP, irrespective of infarct-related artery. These haemodynamic effects are accompanied by only a very modest increase in heart rate during AIVR. Patients with a culprit lesion in the proximal LCA showed a smaller reduction in systolic blood pressure than distal LCA lesions.
Introduction
Accelerated idioventricular rhythm (AIVR) is frequently observed during the reperfusion phase in ST-segment elevation myocardial infarction (STEMI) patients1,2. It is a transient and intermittent accelerated ventricular rhythm with a rate usually between 60 and 110bpm3.
The reported incidence of AIVR in patients undergoing thrombolytic therapy varies between 42 and 88%4-6. Only two studies have reported on the incidence in patients undergoing percutaneous coronary intervention (PCI) for a first STEMI and the data are conflicting7,8. The haemodynamic effects of AIVR have not been reported so far, and it is unknown whether the occurrence and effects of AIVR may be related to the location of the culprit lesion. To address these questions we included 128 consecutive patients with AIVR after successful primary PCI and determined invasive haemodynamic parameters during the procedure.
Methods
Source population
The data analysed in our observational study were obtained from 128 STEMI patients who underwent primary PCI at the Academic Medical Centre, University of Amsterdam. Primary PCI was performed using standard techniques and in accordance with current guidelines. All studied patients were treated with aspirin, clopidogrel and heparin before primary PCI. The use of glycoprotein IIb/IIIa inhibitors during the procedure was left to the discretion of the operator.
Data source
Baseline demographic variables, procedural and angiographic data were prospectively collected in a stand-alone dedicated electronic database at the catheterisation laboratory.
Haemodynamic and electrocardiographic data collection
Throughout the procedure, aortic blood pressure as measured via the guiding catheter, heart rate, and surface 12-lead electrocardiograms were continuously monitored. Surface electrocardiograms were categorised by AIVR morphology to define site of origin using a standard algorithm9.
Patient selection
Patients were selected who had undergone a first primary PCI and showed AIVR following reperfusion. AIVR was defined as a run of >3 consecutive ventricular beats with a rate between 60 and 110bpm. Exclusion criteria were cardiogenic shock at the time of presentation, a previous myocardial infarction or coronary artery bypass surgery, left bundle branch block during sinus rhythm, pacemaker rhythm, supraventricular arrhythmias and ventricular arrhythmias other than AIVR. The study complied with the Declaration of Helsinki.
Data analysis
Aortic pressure and heart rate were analysed using haemodynamic data-acquisition software (MacLab 7000, version 5.2; General Electric Co., Milwaukee, WI, USA). Mean SBP, DBP and heart rate were calculated as the mean of 10 beats during sinus rhythm, and the mean of at least three beats during AIVR. It was accounted for that selected registrations were obtained during stable haemodynamic conditions, without interference of pharmaceuticals (e.g., nitroglycerine).
SBP, DBP and heart rate during AIVR were compared to the values during sinus rhythm. We divided patients according to infarct-related artery and the site of the culprit lesion. The site of the culprit lesion was classified as proximal (right coronary artery, segment1; left anterior descending coronary artery, segment6; or left circumflex coronary artery, segment11) or distal (all other locations).
Statistical analysis
The 2-tailed, paired t test was used for paired data to evaluate differences in blood pressure and heart rate between sinus rhythm and AIVR. The 2-tailed t test was used to compare haemodynamic characteristics between different infarct-related arteries and site of the lesions. Statistical analysis was performed using statistical software package for windows (SPSS, version 16.0.1, 2008; SPSS Inc., Chicago, IL, USA). We consider p<0.05 to be statistically significant.
Results
Patient characteristics
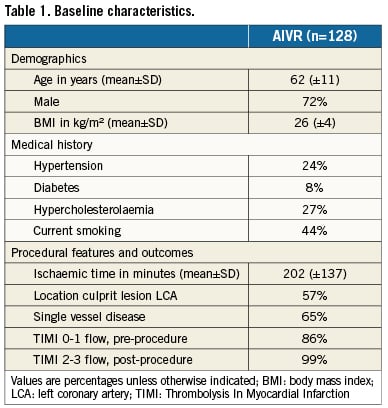
The patient characteristics of our 128 studied patients (aged 62±11 years, 28% women) are shown in Table1. In the AIVR group, two of 129 patients died within 30 days and two additional patients within the first year. The infarct-related artery was the left anterior descending coronary artery in 46%, left circumflex coronary artery in 11% and right coronary artery in 43%.
Haemodynamic parameters
AIVR caused a marked reduction in SBP (130±27 vs. 98±22mmHg, p<0.001) and DBP (80±19 vs.69±16mmHg, p<0.001), as compared to sinus rhythm. Heart rate increased only modestly during AIVR (78±12 vs. 83±11bpm, p<0.001). Figure1 illustrates a typical registration of the decrease in blood pressure during AIVR.
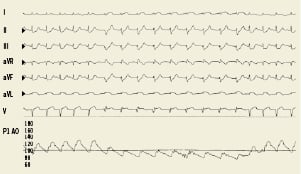
Figure 1. A representative blood pressure registration of accelerated idioventricular rhythm (AIVR) in a ST-segment elevation myocardial infarction patient during primary percutaneous coronary intervention. Note the immediate decrease in blood pressure during an episode of AIVR, as compared to sinus rhythm.
In none of the 128 patients was there a need for the administration of vasopressin or inotropics due to short AIVR duration (mean AIVR duration was 35±25 seconds). We divided the patients into two groups according to the median AIVR duration of 29 seconds. The group with an AIVR duration ≤29 seconds had a mean blood pressure drop of 31±5 mmHg compared to 34±6mmHg in the group with a median AIVR duration of ≥29 seconds (p=NS).
Influence of anatomical site of the lesion
The haemodynamic effects did not depend on the infarct-related artery (Table2). However, reduction in both absolute as well as relative SBP was more pronounced in distal than proximal left coronary artery (LCA) occlusions (36±16 vs. 27±12mmHg, p<0.01, respectively 25±9 vs. 20±8%, p<0.05) (Figure2). This difference in proximal and distal occlusions was not observed in the right coronary artery (121± 27 vs. 124±26 mmHg, p=NS) and (89± 23 vs. 92±21mmHg, p=NS). AIVR had its origin in 92% of the cases in the infarct-related artery zones. The site of AIVR origin did not influence the absolute systolic blood pressure drop (anterior 30±16mmHg, inferior 33±15mmHg, lateral 32±5mmHg and septal 34±15mmHg, p=NS).
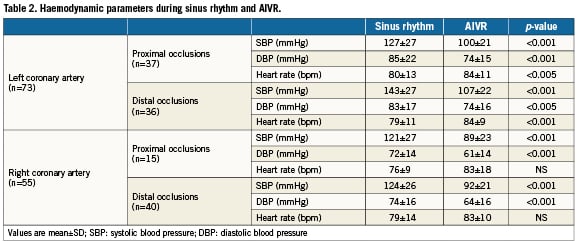
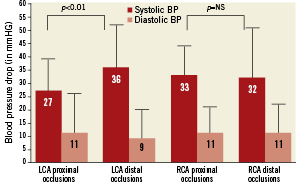
Figure 2. Illustration of the decrease in blood pressure during AIVR, as compared to sinus rhythm, in relation to the site of the coronary lesion. Note that reduction in systolic blood pressure was more pronounced in distal than proximal left coronary artery (LCA) occlusions (36±16 vs. 27±12 mmHg, p<0.01). This difference in blood pressure drop was not observed in right coronary artery (RCA) occlusions.
Discussion
In this study, we are the first to systematically investigate acute haemodynamic effects in STEMI patients with reperfusion-induced AIVR following primary PCI. We found that AIVR following reperfusion is associated with marked reduction in both SBP and DBP, irrespective of infarct-related artery. This reduction in blood pressure is associated with only a very modest increase in heart rate during AIVR. Moreover, patients with a culprit lesion in the proximal LCA show less reduction in blood pressure.
Incidence and mechanisms of AIVR
The reported incidence of AIVR in patients undergoing thrombolytic therapy varies between 42 and 88%4-6. In patients undergoing PCI for STEMI one study reported an incidence of 15% as compared to 42% in a second study7,8. A recent report by Majidi et al showed that reperfusion ventricular arrhythmia (VA) “bursts”, including AIVR, occur more frequently, i.e., in approximately 60 % of the patients. These VA “bursts” occur predominantly within the first 20 minutes following successful reperfusion, illustrating the temporary phenomenon of reperfusion ventricular arrhythmias that are characterised by a rapid onset and disappearance10.
The underlying electrophysiological mechanism of AIVR has been suggested to result from abnormal ventricular automaticity of the subendocardial Purkinje fibres11,12. Kaplinski et al described atransient increase of the idioventricular rate after coronary reperfusion and suggested that this increase in ventricular automaticity may be the basis for the occurrence of AIVR12.
Bonnemeier et al demonstrated that parasympathetic predominance at the sinus node, which was characterised by lower serum norepinephrine levels leading to lower sinus rates, may favour the occurrence of AIVR after reperfusion in acute myocardial infarction patients7.
Reperfusion injury following thrombolysis or primary PCI secondary to calcium overload (and other contributing factors such as formation of free oxygen radicals or changes in intracellular pH) may lead to triggered activity. Some investigators have suggested that triggered activity, based on delayed after depolarisations, may be another explanation of the occurrence of AIVR13,14. An alternative explanation is suggested by previous observations from our institution showing that patients with a pre-existing diastolic dysfunction are more prone to develop non-sustained AIVR15 (Remmelink, MD; unpublished data, 2010).
Prognostic value of AIVR
AIVR has been proposed as a specific non-invasive marker for successful coronary artery reperfusion in the pre-thrombolytic and thrombolytic era2,11. However, in the era of direct mechanical reperfusion strategies, the prognostic relevance of AIVR remains controversial7,8,16. The occurrence of AIVR is generally considered as benign and without prognostic implications7,8, although this topic of interest has not been studied in large-scale clinical studies. One study reports that reperfusion VA bursts, including AIVR, may be used as a marker of reperfusion injury due to the positive association of VA bursts and myocardial infarct size17. In our observational study, both 30-day and one-year mortality rates were low and comparable to mortality rates of patients without cardiogenic shock in the entire PCI population in our institution as well as data from the literature. However due to the small sample size, we cannot exclude different mortality rates in AIVR patients.
AIVR and systemic blood pressure
In our study, we observed a marked decrease in systolic and diastolic blood pressure during an episode of AIVR. An explanation for the immediate decrease in blood pressure may be the absence of atrioventricular sequential pacing. Atrioventricular sequential pacing may lead to optimisation of the timing of the mechanical atrial and ventricular synchrony. Moreover, optimal diastolic filling and reduction of diastolic mitral regurgitation may contribute to the haemodynamic improvement18.
Another explanation may be an absence of atrial contraction (i.e., atrial kick) to left ventricular filling in AIVR19. The significance of atrial contraction to maintain cardiac output has been documented in numerous studies19,20 and is supported by clinical observations showing a marked haemodynamic deterioration in patients developing atrial fibrillation in the setting of ischaemic heart disease21. The occurrence of AIVR was not accompanied by a large increase in heart rate in our study, excluding this as a mechanism of the compromised haemodynamic status during AIVR.
AIVR and proximal versus distal location of culprit lesion
Interestingly, we found that the location of the culprit lesion, i.e., the distal versus the proximal segment of the left coronary artery, was associated with the degree of systolic blood pressure reduction. Patients with a culprit lesion in the distal LCA showed more pronounced reduction in blood pressure than patients with a proximal lesion. Patients with a proximal LCA culprit lesion, and therefore alarger area at risk, had lower blood pressures and concomitant higher heart rates during sinus rhythm. This suggests that patients with a proximal lesion exhibit a more compromised haemodynamic status at baseline and, therefore, probably a less pronounced effect of AIVR on blood pressure than in patients with a smaller area at risk in case of a distal location.
In contrast, patients with a proximal or distal culprit lesion in the RCA show no significant differences of AIVR on blood pressure. The RCA, compared to the LCA, supplies a relatively small area at risk of the left ventricle, independent of the site of the coronary occlusion, which might explain these results.
Conclusion
This is the first study describing that, in STEMI patients treated with PCI, reperfusion-induced AIVR is associated with marked reduction in both SBP and DBP. The occurrence of AIVR is associated with only a very modest increase in heart rate. Moreover, patients with a culprit lesion in the proximal LCA showed less reduction in blood pressure.
Acknowledgement
The authors acknowledge the nursing staff of the cardiac catheterisation laboratory for their skilled assistance.
Conflict of interest statement
The authors have no conflict of interest to declare.