Abstract
Aims: Pressure-controlled intermittent coronary sinus occlusion (PICSO) may improve myocardial perfusion after pPCI. We evaluated the safety and feasibility of PICSO after pPCI for STEMI, and explored its effects on infarct size and myocardial function.
Methods and results: Thirty patients were enrolled following successful pPCI of a left anterior descending coronary artery culprit lesion for anterior STEMI, in whom PICSO for 90 minutes was attempted. Infarct size and myocardial function were assessed by cardiovascular magnetic resonance (CMR) at two to five days and four months post pPCI. An independent core laboratory selected matched historical control patients with CMR data for comparison. PICSO was initiated in 19 patients (63%), and could be maintained for 90 (±2) minutes in 12 patients (40%). Major adverse safety events occurred in one patient (3%). Comparing all PICSO-treated patients to matched controls demonstrated no significant differences in infarct size or myocardial recovery. However, infarct size reduction from two to five days to four months was greater for patients successfully treated with PICSO compared with matched controls (41.6±8.2% vs. 27.7±9.9%, respectively; p=0.04).
Conclusions: PICSO is safe in the setting of STEMI, although feasibility was limited. Administration of sufficient PICSO therapy may be associated with enhanced myocardial recovery during follow-up, warranting further evaluation of this novel therapy.
Introduction
Despite substantial mortality reduction associated with the widespread use of timely primary percutaneous coronary intervention (pPCI) for ST-segment elevation myocardial infarction (STEMI), STEMI survivors remain at high risk for recurrent cardiovascular events. In this regard, the extent of myocardial necrosis is an important determinant of post-infarct cardiovascular events. It is well recognised that successful achievement of epicardial vessel patency by pPCI does not imply optimal restoration of perfusion at the microvascular level, which remains impaired in up to 40% of patients1,2, and is associated with greater infarct size, reduced left ventricular function, and adverse clinical outcome3. Optimisation of myocardial perfusion after pPCI is therefore considered an important therapeutic strategy to limit morbidity and mortality in STEMI survivors4.
Pressure-controlled intermittent coronary sinus occlusion (PICSO) aims to improve microvascular perfusion after pPCI for STEMI by intermittently increasing the pressure in the cardiac venous outflow tract using a balloon-tipped catheter introduced in the coronary sinus5. PICSO is proposed to redistribute venous blood to the border zone of the ischaemic myocardium6-8, to enhance washout of deleterious agents from the microcirculation9, and to induce release of vascular growth factors from the venous endothelium10,11, thereby limiting the extent of myocardial necrosis and enhancing infarct healing. Data from large animal models of acute myocardial infarction show that intermittent occlusion of the coronary sinus yields the potential for a marked reduction in infarct size12, and the underlying haemodynamic effects of PICSO were recently confirmed in a clinical study5. The present study is the first to evaluate the safety and feasibility of PICSO in the setting of acute STEMI, concomitantly exploring its effects on myocardial necrosis and myocardial function.
Methods
STUDY OVERSIGHT
Prepare RAMSES was a prospective, multicentre, non-randomised safety and feasibility evaluation of adjuvant PICSO treatment in patients with anterior wall STEMI treated by pPCI, concomitantly exploring its effects on myocardial function and infarct size by cardiovascular magnetic resonance (CMR) compared with a historical control group. The institutional review board at each participating centre approved the study, and all eligible patients provided informed, written consent. The institutional review board of the VU University Medical Center approved the use of CMR images, clinical data, and procedural data of the selected control patients.
The study sponsor (Miracor Medical Systems GmbH, Vienna, Austria) was responsible for data collection. Data monitoring was performed independently (MedPace Medical Device, Blaine, MN, USA), and data analyses were performed by the investigators. The sponsor had no role in data interpretation or writing of the manuscript.
STUDY DEVICE
The PICSO® Impulse System (Miracor Medical Systems GmbH, Vienna, Austria) comprises an 8 Fr balloon-tipped flexible hypotube catheter (Figure 1A), which is controlled by a dedicated console (Figure 1B). Occlusion of the coronary sinus is achieved by means of ECG-triggered, pressure-controlled balloon inflation, and is maintained until a coronary sinus pressure plateau is reached. Figure 2 shows a typical example of coronary sinus pressure modulation during PICSO.
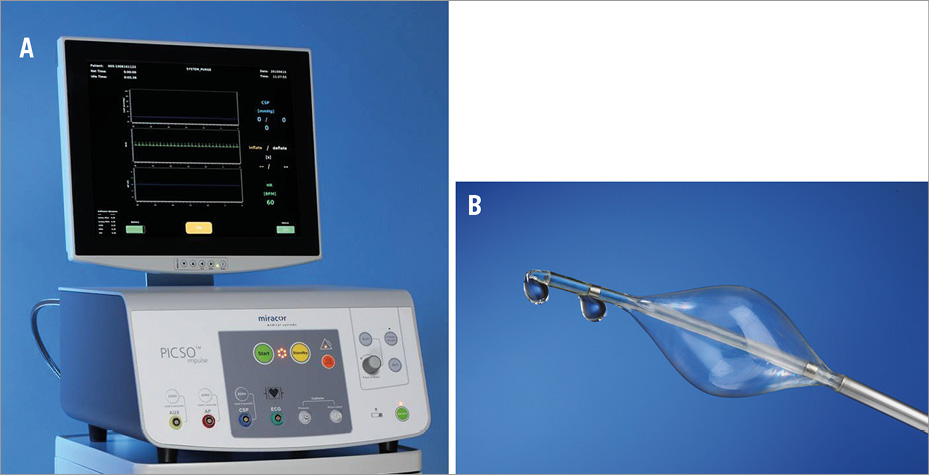
Figure 1. The PICSO Impulse console (A) and PICSO Impulse catheter (B).
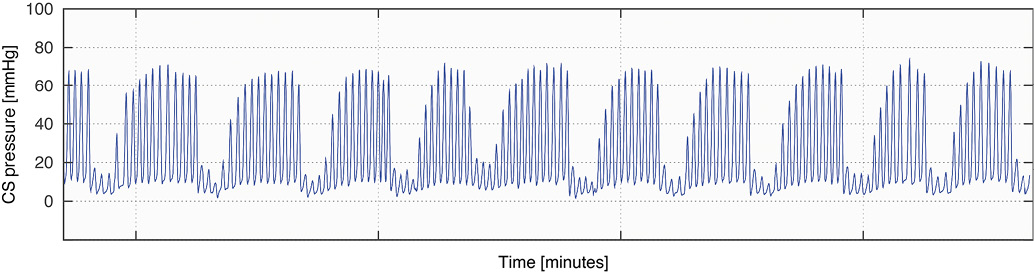
Figure 2. Typical example of the coronary sinus pressure modulation induced by PICSO.
PATIENT POPULATION
Patients aged ≥18 years with a first STEMI, defined by symptoms consistent with STEMI >30 minutes duration but <12 hours, and ≥1 mm of ST-segment elevation in two or more contiguous leads in V1-V4, who underwent uncomplicated pPCI of a single-vessel left anterior descending coronary artery (LAD) culprit lesion were eligible for enrolment. Uncomplicated pPCI was defined as angioplasty followed by stent placement or direct stenting without the occurrence of adverse events, such as major bleeding, coronary perforation, hypotension, pulmonary oedema, or haemodynamic instability. Exclusion criteria included: the presence of a left main coronary artery culprit lesion; previous coronary artery bypass graft surgery; known contraindication to CMR; known creatinine clearance less than 30 mL/min/1.73 m2 or dialysis; haemoglobin level less than 10 g/dL, platelet count less than 100,000 cells/mm3, known coagulopathy or bleeding diathesis; history of stroke, transient ischaemic attack or reversible ischaemic neurological disease within the previous six months; the presence of any lead in the coronary sinus; cardiogenic shock or cardiopulmonary resuscitation; and any comorbid condition likely to interfere with protocol compliance or associated with less than one-year survival.
HISTORICAL CONTROL GROUP
Included patients were matched with a historical control group from the VU University Medical Center CMR database with a CMR scan date between January 2005 and December 2012, which was performed independently by the core laboratory (Image Analysis Center - Cardiology, Amsterdam, The Netherlands). The control group consisted of patients who met the inclusion and exclusion criteria of Prepare RAMSES, and who underwent CMR scanning according to the Prepare RAMSES protocol. All control patients were matched for age, body mass index, coronary segment of the LAD culprit vessel, symptom-to-balloon time, and days from pPCI to CMR scan.
STUDY PROTOCOL
Emergency angiography and pPCI were performed using standard techniques, and according to clinical practice guidelines. Immediately after successful PCI of the LAD culprit lesion, a 9 Fr compatible steerable guide sheath (C.R. Bard Inc., New Providence, NJ, USA) was used to engage the ostium of the coronary sinus using the right femoral venous route, guided by the venous return phase of a left coronary angiogram. The coronary sinus was wired using a 0.032-inch guidewire, after which the PICSO Impulse catheter was advanced into the coronary sinus over-the-wire. Subsequently, the guidewire was exchanged for a 0.014-inch wire, which was left in situ for support, while the guide sheath was retracted into the right atrium (Figure 3, Moving image 1). Procedural anticoagulation was provided to maintain a target activated clotting time of 200-250 seconds for unfractionated heparin. The guide sheath was flushed continuously with heparinised saline (5,000 units in 500 cc 0.9% NaCl at 3 cc/hour). The first 30 minutes of PICSO therapy were provided in the catheterisation laboratory, after which the patient could be transferred to the coronary care unit at the discretion of the operator. Therapy was provided for a maximum of 90 minutes in total, after which the Impulse catheter was removed.
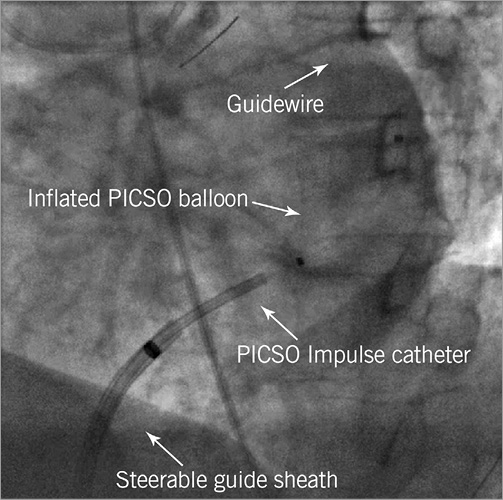
Figure 3. PICSO Impulse catheter position (Moving image 1).
CMR was scheduled in all patients at two to five days after pPCI, as well as at four-month follow-up. All patients were treated with aspirin indefinitely and with a P2Y12 inhibitor for at least one year.
CARDIOVASCULAR MAGNETIC RESONANCE PROTOCOL AND DATA ANALYSES
Cine images were acquired for measurement of global left ventricular (LV) volumes and function, and segmental wall thickness. Delayed-enhancement (DE) images were acquired for measurement of the size and extent of infarction and microvascular obstruction (MVO), and to delineate the infarct endocardial surface area (ESA). The area at risk was measured using the ESA. Myocardial salvage index (MSI) was calculated as the difference between the area at risk and the total infarct size, normalised for area at risk. All CMR data were analysed by the core laboratory on a separate workstation using validated software (QMass® 7.6; Medis, Leiden, The Netherlands), blinded to patient identity and clinical information. A full description of the CMR protocol and analyses is presented in the Online Appendix.
CORONARY SINUS PRESSURE DATA ANALYSIS
Coronary sinus pressure data were analysed offline to calculate PICSO quantity, defined as: balloon inflation hold time*(mean systolic coronary sinus pressure plateau - mean coronary sinus pressure during deflation)*(mean systolic coronary sinus pressure plateau - mean diastolic coronary sinus pressure plateau), summed over the complete PICSO procedure and expressed in (mmHg). PICSO quantity reflects the magnitude of coronary sinus pressure modulation during the PICSO procedure, and is a marker of PICSO therapy performance.
ENDPOINTS AND DEFINITIONS
The primary endpoint was feasibility of PICSO in STEMI, defined as successful delivery of the PICSO Impulse catheter and successful administration of PICSO treatment for 90 minutes. Safety of PICSO was assessed as the rate of serious adverse device events, defined as any major complications or death caused by the implantation procedure or the presence or performance of the device. Additionally, the occurrence of major adverse cardiac events (MACE) was documented, defined as the composite of all-cause death, myocardial reinfarction not clearly attributable to a non-target vessel, or new onset of severe heart failure or hospitalisation for heart failure.
Exploratory CMR endpoints included infarct size, MSI and MVO, as well as LV volumes and function at two to five days post pPCI, and at four-month follow-up.
STATISTICAL METHODS
Enrolment was planned for 40 patients to obtain meaningful safety and feasibility information, while minimising unnecessary patient exposure. Considering the small sample size, parametric testing was used for the principal analysis of the exploratory CMR endpoints. Continuous descriptive variables are presented as mean and standard deviation, or median and first and third quartiles, and are compared by Student’s t-test or Wilcoxon rank-sum test, as appropriate. For segmental wall thickening analyses, a linear mixed-effects model with robust standard errors was used to account for clustering of LV segments within patients. All statistical tests were two-sided, and a p-value <0.05 was considered significant. All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX, USA).
Results
PATIENTS
Between January 2012 and June 2013, patients with STEMI were screened at six sites in four countries (The Netherlands, Germany, Switzerland, and Austria). Enrolment was halted after inclusion of 30 patients due to slow enrolment and a relatively high rate of unsuccessful PICSO procedures. Baseline and procedural characteristics appear in Table 1.
PICSO FEASIBILITY
PICSO therapy was initiated in 19 out of 30 patients (63%), and was provided for the intended 90 (±2) minutes in 12 patients (63% of patients in whom PICSO could be initiated, 40% of all included patients).
In the 11 patients in whom PICSO therapy was not initiated, this resulted from the inability to engage the coronary sinus in one patient (3%), from a user error in one patient (3%), from an unstable PICSO catheter position prohibiting catheter calibration in six patients (20%), and from exceeded PICSO console safety limits in one patient (3%). A serious adverse event during PICSO catheter calibration precluded initiation of PICSO therapy in one patient (3%), as described in detail below. Additionally, the study protocol was aborted prior to PICSO initiation in one patient (3%) as a result of catheterisation laboratory logistics.
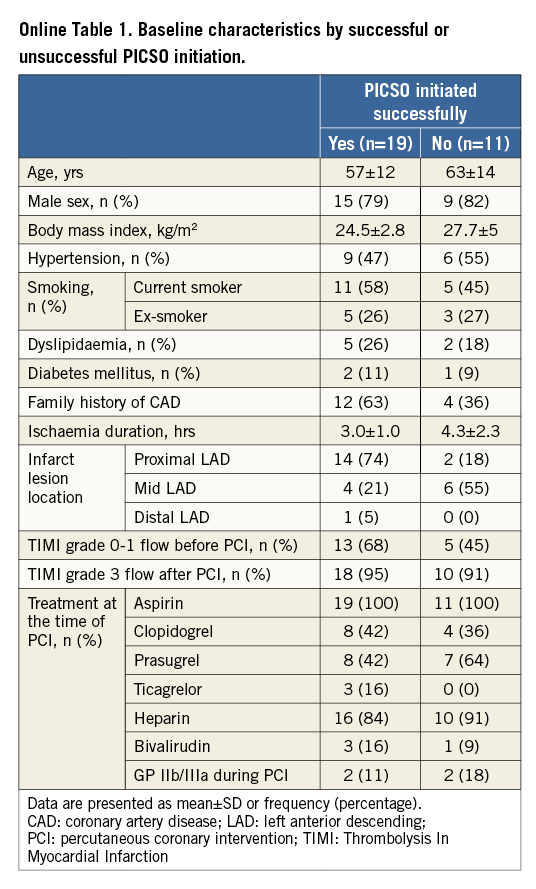
In the seven patients in whom maintenance of PICSO therapy for 90 (±2) minutes was unsuccessful, this resulted from catheter instability in two patients (7%), and from exceeded PICSO console safety limits in four patients (13%). Additionally, PICSO therapy was aborted in one patient (3%) as a result of catheterisation laboratory logistics. Baseline and procedural characteristics stratified by successful PICSO initiation appear in Online Table 1.
PICSO QUANTITY
In those patients in whom PICSO was initiated, PICSO therapy was provided for a median of 88.8 minutes (Q1, Q3: 72.0, 89.6 minutes). Median PICSO quantity in these patients was 494 mmHg (Q1, Q3: 160, 1,171 mmHg). PICSO quantity was substantially higher in the centre with previous experience with the PICSO Impulse System (AMC-UvA), as compared to the other centres (852 mmHg [Q1, Q3: 572, 1,347 mmHg] vs. 160 mmHg [Q1, Q3: 82, 282 mmHg], respectively; p=0.007), despite equivalent PICSO therapy duration across centres (88.9 minutes [Q1, Q3: 81.3, 90.6 minutes] vs. 88.8 minutes [Q1, Q3: 72.0, 89.2 minutes], respectively; p=0.46).
PICSO SAFETY
One serious adverse device event was reported during the study. In this patient, cardiac tamponade occurred immediately after starting PICSO calibration, in retrospect originating from guidewire-induced coronary sinus perforation during wiring of the coronary sinus. As such, the adverse event was attributed to the implantation procedure. The tamponade was successfully managed with pericardiocentesis. Nonetheless, the patient developed segmental pulmonary embolism during hospitalisation, for which oral anticoagulation was prescribed for six months post discharge without further clinical sequelae.
Three patients were transferred to the coronary care unit to receive the final 60 minutes of PICSO therapy without the occurrence of adverse events. During follow-up, two episodes of MACE occurred. One patient in whom PICSO could not be initiated died seven months after the procedure without documented cause, and one patient in whom only low PICSO quantity was achieved died nine months after the procedure due to a major stroke.
EFFICACY ANALYSIS OF PICSO VERSUS HISTORICAL CONTROL PATIENTS
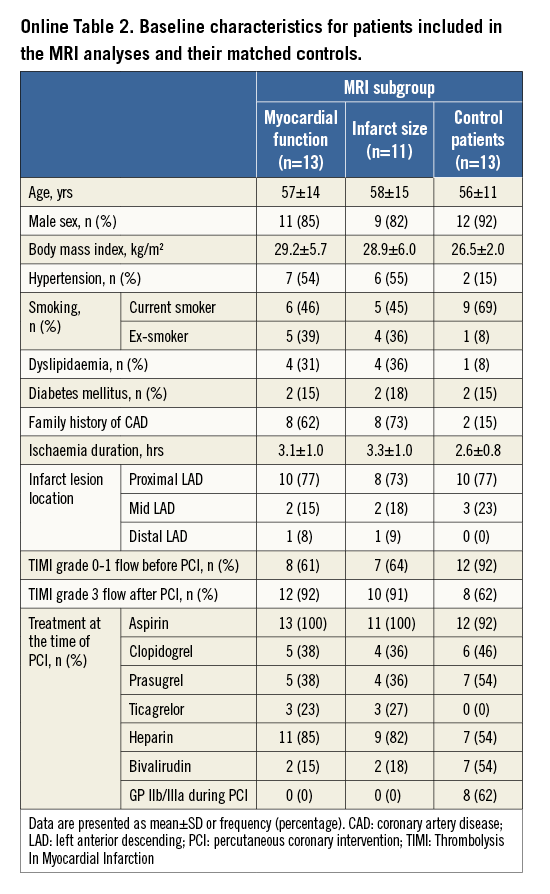
Evaluable CMR data were available in 13 PICSO patients for global and segmental LV function, and in 11 PICSO patients for infarct size analyses. The baseline characteristics of these patients appear in Online Table 2.
Global and segmental LV function at baseline and follow-up CMR were not significantly different in PICSO patients compared with their matched controls (Table 2). Similarly, infarct size at baseline and follow-up, the extent of MVO, and the reduction in infarct size from baseline to follow-up were not significantly different between the groups (Figure 4A, Table 2).
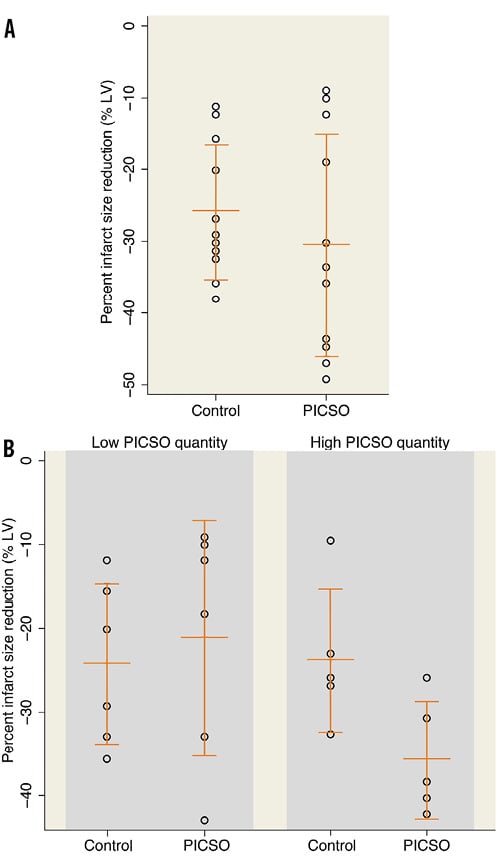
Figure 4. Infarct size reduction between: A) PICSO and matched historical control patients, and B) PICSO and matched historical control patients, stratified by high or low PICSO quantity. Infarct size reduction is expressed as the percent change in infarct size (% of the left ventricle) from baseline (day 2-5) to four-month follow-up.
CMR ANALYSES ACCORDING TO HIGH OR LOW PICSO QUANTITY VERSUS MATCHED CONTROL PATIENTS
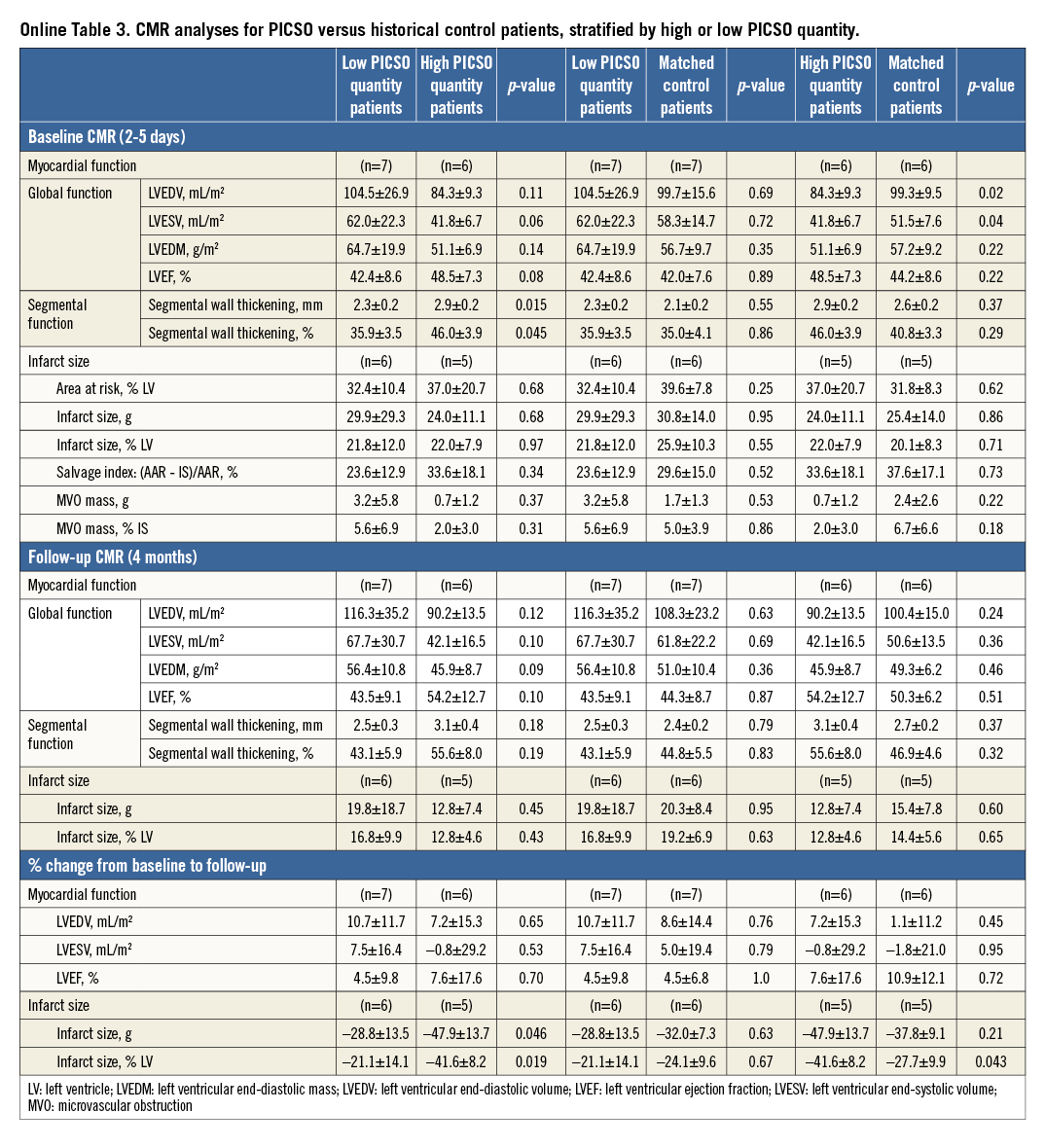
Since efficacy of PICSO conceivably depends on effective modulation of coronary sinus pressure, and is probably negligible when pressure modulation is minimal, additional CMR analyses were performed according to the magnitude of PICSO quantity. In the absence of clinical cut-off values for PICSO quantity, patients were stratified into a high and low PICSO quantity group based on the median PICSO quantity (494 mmHg).
Myocardial function tended to be preserved to a greater extent in patients with high as compared to low PICSO quantity (Online Table 3). Moreover, high PICSO quantity was generally associated with favourable trends in the magnitude of infarct size, MVO, and MSI (Online Table 3). Even in this small patient cohort, infarct size reduction from baseline to follow-up MRI was significantly higher for high PICSO quantity patients compared with those patients in whom PICSO quantity was low (41.6±8.2% vs. 21.1±14.1%, respectively; p=0.02) (Figure 4B, Online Table 3), where the reduction in infarct size from baseline to follow-up CMR showed a significant dose dependency (r2=0.70, p=0.008) (Figure 5).
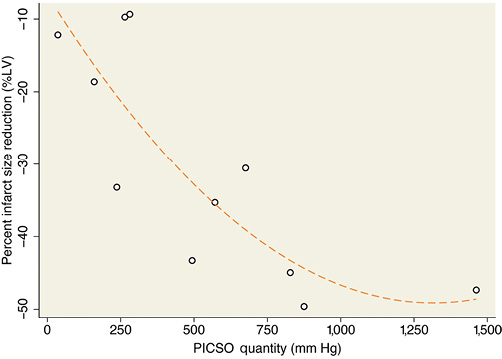
Figure 5. Relationship between PICSO quantity and infarct size reduction expressed as the percent change in infarct size (% of the left ventricle) from baseline (day 2-5) to four-month follow-up.
These favourable trends for patients with high PICSO quantity were confirmed as compared to their matched control patients (Online Table 3). Notably, infarct size reduction from baseline to follow-up CMR was significantly greater in the high PICSO quantity group compared with its matched controls (41.6±8.2% vs. 27.7±9.9%, respectively; p=0.04) (Figure 4B, Online Table 3). MVO tended to be reduced in the high PICSO quantity group compared to the matched control group, although this difference did not reach statistical significance (2.0±3.0% versus 6.7±6.6% of total infarct size, respectively; p=0.18). In contrast, the low PICSO quantity patients demonstrated similar LV function and volumes at baseline and follow-up, and similar MVO and infarct size reduction over time compared to their matched control patients (Figure 4B, Online Table 3).
Discussion
In this prospective, non-randomised, multicentre, safety and feasibility study involving patients with anterior STEMI who underwent successful pPCI of a LAD culprit lesion, adjuvant treatment with the PICSO Impulse System was safe, although successful PICSO Impulse catheter positioning, initiation and maintenance of PICSO therapy was limited to 40% of patients. Nonetheless, successful PICSO therapy administration was associated with favourable trends in CMR infarct size and myocardial function parameters, compared both to patients in whom PICSO administration was unsuccessful, as well as to matched control patients. Notably, successful PICSO administration was associated with a significant improvement in myocardial recovery from two to five days to four months post pPCI as compared to patients in whom PICSO administration was unsuccessful, as well as to matched control patients.
PICSO SAFETY AND FEASIBILITY IN ANTERIOR WALL STEMI
The use of the PICSO Impulse System was generally safe, with only one major adverse safety event which was related to the implantation procedure – similar to the implantation-related complications observed in routine electrophysiology procedures13. PICSO therapy was not initiated in 37% of included patients, which was largely attributable to technical difficulties with catheter stability, precluding appropriate calibration of the PICSO catheter. In patients in whom PICSO was initiated, PICSO could be provided for the intended 90 (±2) minutes in 63% of patients, which was largely dictated by the strict PICSO Impulse console safety settings. These safety limits apply for example to heart rate, as well as to intraluminal and balloon pressures in the PICSO catheter, which preclude safe functioning of the device, and lead to an instant abortion of PICSO therapy or its initiation.
The provided PICSO quantity varied markedly from 15 to 2,735 mmHg, and was substantially higher in patients treated within the most experienced centre, despite equivalent PICSO therapy duration across centres, which suggests an important learning curve required for successful application of the technique. The anticipated mechanisms of action of PICSO are based on the mechanical effects of intermittent pressure increase in the cardiac venous outflow tract. Therefore, suboptimal administration of PICSO, as documented by low PICSO quantities, is conceivably associated with limited therapeutic efficacy, which is in line with the dose dependency of PICSO in the present study. The successful modulation of coronary sinus pressure during PICSO is related to both catheter positioning and stability. Catheter positioning near the coronary sinus ostium provides the largest magnitude of venous inflow, and therefore the largest modulation of coronary sinus pressure. Catheter stability during balloon occlusion is better in more proximal, narrower parts of the coronary sinus, where the distending pressure of the balloon against the coronary sinus wall is larger, securing the catheter to the coronary sinus wall despite substantial backward pressure. The feasibility results of the Prepare RAMSES study indicate that optimal positioning of the catheter is subject to a learning curve, and that current PICSO technology may benefit from improvements to allow reliable and optimal therapeutic efficacy in patients in daily clinical practice.
CARDIOVASCULAR MAGNETIC RESONANCE ANALYSES
Although by intent-to-treat there was no significant reduction in infarct size with PICSO compared to control, administration of high PICSO quantity was associated with favourable trends in myocardial function and infarct size parameters, when compared both to those patients in whom administered PICSO quantity was limited, as well as to matched historical control patients. Administration of high PICSO quantity was also associated with significantly greater myocardial recovery during follow-up by both assessments. These favourable trends were not observed in patients treated with low PICSO quantity. Although no definite conclusions can be drawn from these findings in this small, non-randomised study without a concurrent control, overall the magnitude of effect appears comparable to that with cardio protection during pPCI for STEMI in recent randomised trials14, warranting further evaluation of PICSO in larger efficacy studies.
Limitations
Since the mechanical effects of coronary sinus occlusion are anatomically limited to the left coronary artery, and dominant in the LAD, we limited enrolment to patients with a LAD culprit lesion. Nonetheless, no restrictions were made with respect to proximal or distal lesion location, or with respect to Thrombolysis In Myocardial Infarction flow grade before pPCI. As such, patients may have been included in whom the benefit of adjuvant PICSO therapy after timely pPCI is limited. Moreover, Prepare RAMSES was not powered for assessment of therapeutic efficacy, increasing the risk of type I error and overestimation of effect size in the CMR efficacy analyses. In consideration of the small sample size and the use of a historical control group instead of randomised patient allocation, these results should be considered hypothesis-generating, pending the performance of an adequately powered randomised trial.
Conclusion
Pressure-controlled intermittent coronary sinus occlusion using the PICSO Impulse System was safe in patients with anterior STEMI, and exploratory efficacy analyses suggest that PICSO may augment myocardial recovery after primary PCI for LAD infarction in a dose-dependent manner. These results warrant further exploration of the safety and efficacy of adjuvant PICSO therapy.
| Impact on daily practice Even after timely epicardial reperfusion for ST-segment elevation myocardial infarction (STEMI), microvascular reperfusion remains impaired in up to 40% of patients, which is associated with greater infarct size, reduced left ventricular function, and adverse clinical outcome. Pressure-controlled intermittent coronary sinus occlusion (PICSO) is a novel tool that aims to augment microvascular perfusion in myocardial infarction (MI) patients. We document that, though feasibility was limited, PICSO can be applied safely in STEMI patients, and may be associated with improved myocardial healing at follow-up. If these results can be confirmed using the second-generation PICSO system, which aims to improve feasibility of PICSO, routine application of PICSO in clinical practice may impact on STEMI treatment. However, larger randomised studies are awaited to document whether PICSO improves clinical outcomes. |
Funding
Prepare RAMSES was funded by Miracor Medical Systems GmbH, Vienna, Austria.
Conflict of interest statement
T. van de Hoef, J. Piek, G. Stone, and A. Khattab have provided consultancy services for Miracor Medical Systems. The other authors have no conflicts of interest to declare.
Methods
EXTENDED CARDIAC MAGNETIC RESONANCE IMAGING PROTOCOL AND DATA ANALYSIS
The CMR examination was performed on a 1.5 Tesla clinical scanner (MAGNETOM® Sonata [n=5], MAGNETOM® Avanto [n=20] or MAGNETOM® Aera [n=6]; Siemens, Erlangen, Germany), using electrocardiographic gating and a phased array cardiac receiver coil. Cine images were acquired using a steady state free-precession pulse sequence in consecutive short-axis orientations to cover the left ventricle (LV), for measurement of global LV volumes and function, and segmental wall thickness.
Typical in-plane resolution was 1.6×1.6×5.0 mm3 (slice gap 5 mm, repetition time/echo time=3.2/1.5 ms, flip angle 55-75°, temporal resolution 35-45 ms). Late gadolinium enhancement (LGE) images were acquired for measurement of the size and extent of infarction and microvascular injury (MVI), and to delineate the infarct endocardial surface area (ESA). A two-dimensional segmented inversion recovery gradient-echo pulse sequence was used 10-15 min after the administration of a gadolinium-based contrast agent (0.2 mmol/kg), with identical slice position to the cine images. Typical in-plane resolution was 1.5×1.5×5.0 mm3 (slice gap 5 mm, repetition time/echo time=9.6/4.4 ms, flip angle 25°, inversion time=240-300 ms, triggering to every heartbeat).
All CMR data were analysed on a separate workstation using commercial software (QMass® 7.6; Medis, Leiden, The Netherlands), and were evaluated blinded to patient identity and clinical information by the core laboratory (Image Analysis Center - Cardiology, VU University Medical Center, Amsterdam, The Netherlands). On all short-axis cine images, the endocardial and epicardial borders were outlined manually on the end-diastolic and end-systolic images, to measure LV volumes and calculate ejection fraction (EF). Segmental LV function was determined automatically according to the standard 17-segment AHA/ACC model15, to measure end-diastolic and end-systolic wall thickness and calculate wall thickening. Due to partial volume effects and longitudinal shortening of the heart, segment 17 (apex) was excluded from the segmental analysis. Total infarct size was measured by planimetry from the short-axis LGE images, using the full-width-half-at-maximum method, and is expressed as percentage of the end-diastolic LV mass. The transmural extent of infarction was calculated by dividing the hyperenhanced area by the total area of the predefined segment, using the identical segmentation of the cine images. Microvascular injury was identified on the LGE images as hypointense regions within the hyperenhanced myocardium, and was included in the total infarct size16. The area at risk was measured using the infarct endocardial surface area as described by Ortiz-Peréz et al17, as the summed endocardial hyperenhanced infarct length divided by the total LV endocardial length on all consecutive short-axis images, and expressed as percentage. Myocardial salvage index (MSI) was then calculated as the difference between the area at risk and the total infarct size, divided by the area at risk.
Online data supplement
Moving image 1. PICSO balloon inflation/deflation cycle.

