Abstract
Aims: To investigate a pressure-controlled intermittent coronary sinus occlusion (PICSO) system in an ischaemia/reperfusion model.
Methods and results: We randomly assigned 18 pigs subjected to 60 minutes ischaemia by left anterior descending (LAD) coronary artery balloon occlusion to PICSO (n=12, groups A and B) or to controls (n=6, group C). PICSO started 10 minutes before (group A), or 10 minutes after (group B) reperfusion and was maintained for 180 minutes. A continuous drop of distal LAD pressure was observed in group C. At 180 minutes of reperfusion, LAD diastolic pressure was significantly lower in group C compared to groups A and B (p=0.02). LAD mean pressure was significantly less than the systemic arterial mean pressure in group C (p=0.02), and the diastolic flow slope was flat, compared to groups A and B (p=0.03). IgG and IgM antibody deposition was significantly higher in ischaemic compared to non-ischaemic tissue in group C (p<0.05). Significantly more haemorrhagic lesions were seen in the ischaemic myocardium of group C, compared to groups A and B (p=0.002). The necrotic area differed non-significantly among groups.
Conclusions: PICSO was safe and effective in improving coronary perfusion pressure and reducing antibody deposition consistent with reduced microvascular obstruction and ischaemia/reperfusion injury.
Introduction
Prompt recanalisation of the infarct-related artery (IRA) by either thrombolysis or percutaneous coronary intervention (PCI) to reperfuse the jeopardised myocardium is considered the cornerstone of therapy in acute ST-elevation myocardial infarction (STEMI) to reduce morbidity and mortality. Despite its proven ability to restore epicardial coronary blood flow, adequate reperfusion on the myocardial level cannot be accomplished in up to 30-50% of patients with STEMI1. This relates to the development of myocardial microvascular dysfunction as a consequence of the primary epicardial event and/or of the reperfusion itself2. Reperfusion may aggravate tissue damage, so-called “ischaemia/reperfusion injury”3, where the complement system interacts with the endothelium, producing this damage4.
Intermittent coronary sinus occlusion (ICSO), introduced several decades ago, comprises intermittent pressure increase in the coronary sinus and was evaluated in animal models5-7 and small clinical observations8,9. Pressure-controlled intermittent coronary sinus occlusion (PICSO) is a modification of the original time-dependent technology, and is applied through a dedicated catheter system. A potential indication is STEMI, as an adjunct to recanalisation therapy, to improve regional myocardial function of the ischaemic/reperfused myocardium. The proposed mechanism of action is an intermittent pressure increase in the coronary venous circulation leading first to a suction effect (upon release), which may enhance wash-out of noxious and embolic material across the microvascular bed10, and second to retrograde perfusion via the distal IRA and an augmented collateral inflow from adjacent non-IRAs supplying the border zone of the myocardium at stake11.
We sought to investigate a PICSO device in a closed-chest porcine ischaemia/reperfusion model with respect to: safety and feasibility of PICSO application in the setting of infarction, effects of PICSO during ischaemia and reperfusion, and differences related to time of onset of PICSO application.
Methods
STUDY DESIGN
Prospective, randomised, controlled, large animal experiment (house swine, average weight=60-70 kg) investigating PICSO in the setting of ischaemia (60 minutes) and subsequent reperfusion (180 minutes). Eighteen animals were divided into three groups, 12 PICSO (groups A and B) and six controls (group C). The PICSO group was divided into two equal arms depending on the starting time of PICSO intervention in relation to reperfusion. Randomisation was done prior to the experimental series, using a randomisation code created by a random number generator distributing animals into three equal groups. Care and use of animals in this study were according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and in agreement with the Swiss Animal Welfare Law. The local ethics committee for animal research (Amt für Landwirtschaft und Natur des Kantons Bern) approved the study.
DEVICE UNDER INVESTIGATION
The PICSO system used (PICSO Impulse System; Miracor Medical Systems GmbH, Vienna, Austria) is composed of a catheter connected to a console (Figure 1). The catheter contains four lumens, a 15.5 mm in diameter ×20 mm long balloon at the distal end, and connectors on the proximal end for gas supply to the balloon and for the coronary sinus pressure measurement. Pressing START, the device starts with a pressure set up and baseline ICSO. PICSO commences automatically and continues for 30 minutes. Each PICSO cycle starts with controlled balloon inflation triggered by the QRS complex and depends on the pressure reached in the coronary sinus till reaching a plateau, then occlusion is intermittently released. Inflation hold time is limited to a maximum of 30 seconds. The deflation phase between two occlusions lasts at least three seconds. According to the assigned group, PICSO application was installed in group A 10 minutes before, and in group B 10 minutes after the onset of reperfusion. PICSO was applied for 180 minutes of reperfusion in both groups. A further 10 minutes of reperfusion (till 190 minutes) were without PICSO. In group C reperfusion occurred after 60 minutes of ischaemia for 190 minutes without PICSO application.
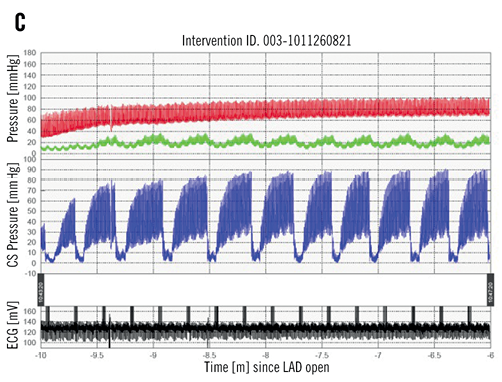
Figure 1. The Miracor PICSO device system: A) console; B) inflated catheter balloon tip, and C) phasic pressure tracing from coronary sinus during PICSO application in a myocardial ischaemia/reperfusion animal model (red: arterial blood pressure; green: distal left anterior descending coronary artery (LAD) pressure; blue: coronary sinus pressure).
CLOSED-CHEST PORCINE ISCHAEMIA/REPERFUSION MODEL
Under intubation anaesthesia, bilateral common carotid artery and internal jugular vein exposure was done by surgical cutdown, followed by direct puncture using Seldinger’s technique. Coronary sinus intubation with the 9 Fr pre-shaped delivery sheath was performed in all animals using a C-arm x-ray unit and contrast dye to identify proper PICSO catheter insertion which was guided over a 0.025” wire (in groups A and B) into the coronary sinus, with the proximal balloon marker situated close to its ostium. Through a standard 6 Fr Judkins left guiding catheter a pressure wire (Radi; St. Jude Medical, St. Paul, MN, USA) was introduced into the distal left anterior descending coronary artery (LAD) after successful calibration. To create ischaemia, the LAD was occluded just distal to the first large diagonal branch by a conventional over-the-wire (3.5 x 15 mm) angioplasty balloon for 60 minutes. The occlusion was confirmed by distal pressure measurements as well as angiographic control at the beginning and before the end of the occlusion. At the end of the experiment (after completion of the reperfusion period), LAD re-occlusion and intravenous injection of 120 mL Evan’s Blue (2% wt/vol. solution) were carried out, leaving all but the area at risk (AAR) stained blue. The animals were sacrificed and immediate cardiac explantation was performed.
TEST SYSTEM JUSTIFICATION
The size and anatomy of the cardiovascular system of healthy large swine are clinically relevant for testing such a medical device. Nevertheless, there is one notable difference in coronary venous drainage between species, namely that the left azygous vein draining the left thoracic cavity directly joins into the coronary sinus in the pig’s heart, which renders placement of the balloon catheter distal to its anastomosis in the coronary sinus essential to generate sufficient pressure in the coronary venous circulation. Furthermore, the coronary arterial circulation in swine is deficient of collaterals, where each area of myocardium is supplied by a single coronary artery. Therefore, collateral flow contribution to the border zone of the area at risk, which is an important mechanism of limiting infarct size in humans, cannot be examined in such a model.
DATA ACQUISITION AND ANALYSIS
HAEMODYNAMICS
Left ventricular function
A 7 Fr admittance pigtail catheter was inserted via the right carotid artery into the left ventricle to acquire left ventricular pressure-volume (PV) loops. The data were analysed by selecting 30 sequential beats at each time point.
Myocardial perfusion
The following parameters were derived to assess microvascular dysfunction12,13: coronary pressure measured during balloon occlusion of the LAD at 10 and 50 minutes of ischaemia and at 190 minutes of reperfusion (coronary wedge pressure). The diastolic flow slope was drawn between the LAD diastolic pressure at 180 minutes reperfusion and the coronary wedge pressure at 190 minutes. Diastolic flow slope is indicative of the forward driving pressure across the microvascular bed. A flat slope (small pressure difference) indicates poor forward flow, while a steep slope (high pressure difference) indicates a good flow. Last, the difference between distal LAD and systemic arterial mean pressures at the beginning and end of reperfusion was recorded.
STAINING FOR MYOCARDIAL INFARCT SIZE
The investigators, including the pathologists performing subsequent staining and histological/immunochemical analysis, were blinded with regard to treatment assignment. The heart was cut perpendicular to the septum from the apex to the base into 3 mm slices until the mitral valve. Entire slices were stained with 1% triphenyl tetrazolium chloride (TTC). Viable myocardium (viable ischaemic tissue [VIT]) was stained bright red while the infarcted tissue (necrotic ischaemic tissue [NIT]) remained unstained. Evan’s Blue-stained areas of the left ventricle were expressed as a percentage of the left ventricle. This gave a measure of the %AAR of the left ventricle. The TTC stained and unstained areas within the AAR were marked and expressed as percentages of the AAR to obtain the amount of viable tissue and necrotic tissue (infarct size), respectively.
IMMUNOFLUORESCENCE STAINING OF THE TISSUE
Complement deposition was assessed using rabbit anti-human polyclonal C3c antibody, and with Cy3-labelled sheep anti-rabbit as secondary antibody. Natural antibody deposition was assessed with the help of goat anti-porcine IgG FITC and IgM FITC antibodies. Area under the curve (AUC) value was obtained from the normalised histogram to derive a final score for the section. Sections were then compared with their final scores.
HAEMATOXYLIN AND EOSIN (H&E) STAINING OF MYOCARDIAL TISSUE
Scores were given to H&E stained slides from each tissue type (area not at risk [ANR], VIT, NIT). The scores were given from 0-3 where 0 is the least and 3 is the maximum. Analysis included myocyte damage determined by presence of contraction bands and cell necrosis, tissue oedema based on spaces between muscle bundles and cells, and haemorrhagic lesions.
SOLUBLE MARKERS MEASURED IN CORONARY SINUS BLOOD
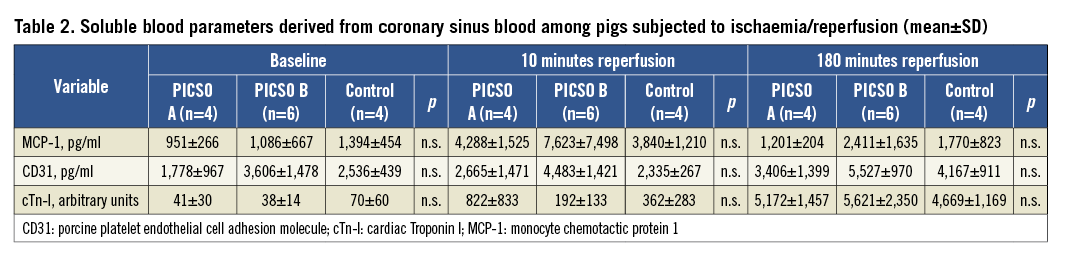
Coronary sinus blood serum samples were taken before LAD occlusion, 50 minutes after, and every 30 minutes of reperfusion. Commercially available antibodies specific for porcine platelet endothelial cell adhesion molecule (CD31), cardiac Troponin (cTn-I), monocyte chemotactic protein (MCP-1) were used as capture antibodies by coupling them to different fluorescent signatures of polystyrene COOH Luminex beads by using a Bio-Plex amine coupling kit.
STATISTICAL ANALYSIS
Infarct size data were compared using one-way analysis of variance (ANOVA). For haemodynamic, blood parameter and staining data, one-way analysis of variance (ANOVA) with post hoc Dunnett’s t-test was used to detect significant differences among the three treatment groups for a certain time point whereas two-way repeated-measures ANOVA for repeated measures was applied to determine the significance of effect of time and treatment as fixed parameters. For direct comparison between two treatment groups for a certain time point two-sample t-tests assuming unequal variances were used. Differences were considered statistically significant at a p-value <0.05. All p-values are results of two-tailed tests. Microsoft Office Excel 2007 version 12.0 (Microsoft Corp., Redmond, WA, USA) software and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) were used for statistical analyses. Data, unless otherwise specified, are presented as mean±standard deviation in the text, Tables and Figures. Six pigs did not complete the experiments: two died during the induction of myocardial infarction due to intractable ventricular fibrillation, in three pigs coronary sinus cannulation was not possible, and in one pig coronary sinus perforation and pericardial tamponade occurred during introduction of the delivery sheath into the coronary sinus. These six pigs were replaced by six other pigs to complete the predefined total of 18 pigs. There was random assignment of the 18 pigs into three equal groups (n=6, each). However, in one pig allocated to group B, PICSO was erroneously started 10 minutes before reperfusion. Therefore, since data analysis is based on actual treatment received, this pig was included in group A. Poor quality recordings were excluded from analysis to avoid bias.
Results
PICSO SAFETY AND FEASIBILITY
Mean PICSO inflation hold time per cycle was 14.3±5.8 seconds for group A and 14.9±4.5 seconds for group B. Coronary sinus pressure reflected the onset and course of PICSO application as per the designated treatment group and compared to the control group, in which no notable change in coronary sinus pressure throughout the experiment was seen. Figure 2 shows the coronary sinus mean pressure along the time course of the experiment in the three groups. The coronary sinus pressure during inflation hold time was 60.0±8.5 mmHg for group A and 63.4±5.6 mmHg for group B, and 14.6±4.9 mmHg for group A and 20.6±4.6 mmHg in group B during deflation time. Out of all PICSO cycles, 3.9% only reached the safety limit of 30 seconds and inflation was interrupted accordingly. No technical difficulties necessitating treatment abortion were encountered. At autopsy, the coronary sinus which was longitudinally opened showed no macroscopic evidence of thrombi or perforations in any animal.
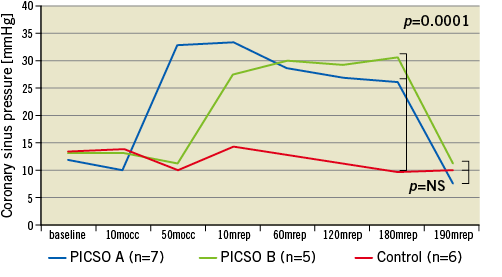
Figure 2. Coronary sinus pressure changes over the time course of the experiments in the three study groups. mocc: minutes occlusion; mrep: minutes reperfusion
HAEMODYNAMICS
Left ventricular function
Table 1 displays the absolute values of the measured parameters at selected time points.
Myocardial perfusion
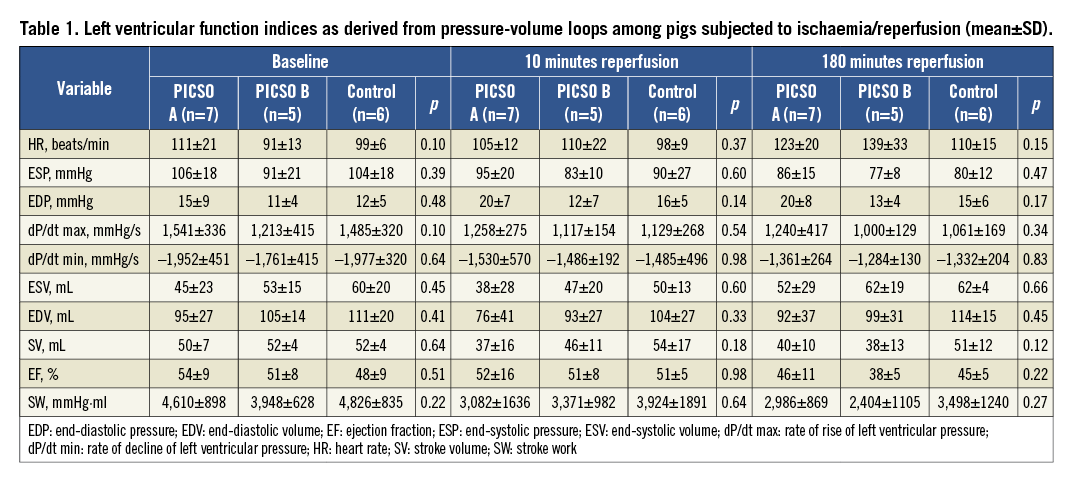
Distal LAD pressure continuously declined along the course of reperfusion in group C to become significantly lower at its end compared to group A. This pressure drop separately involved the diastolic (p=0.02), systolic (p=0.03) and mean (p=0.05) pressure at 180 minutes reperfusion (Dunnett’s t-test for all three significant at α=0.05). Figure 3 plots the LAD pressure changes across the time course of the experiments among all groups. These differences in LAD pressure were witnessed in spite of a constant systemic arterial mean pressure in all groups throughout the experiments. During reperfusion, LAD pressure was not significantly lower with regard to systemic arterial mean pressure in all groups till 60 minutes, after which LAD pressure dropped in group C to become significantly lower than arterial blood pressure till the end of reperfusion (p=0.02) (Figure 4). The coronary wedge pressure was very low at 10 minutes ischaemia (4.5 vs. 4.8 vs. 5.2 mmHg, for groups A, B and C, respectively), while at 50 minutes ischaemia, i.e., at PICSO onset in group A, there was a significant rise in wedge pressure in this group (15.7 vs. 5.7 vs. 6.6 mmHg, for groups A, B and C, respectively). At the end of reperfusion, the wedge pressure was higher in groups C and B (9.6 and 10.4 mmHg, respectively) compared to group A (6.2 mmHg). Accordingly, the diastolic flow slope showed a significant difference among groups, being more flat in group C compared to A (p=0.03) and compared to B (p=0.03) (Figure 3C).
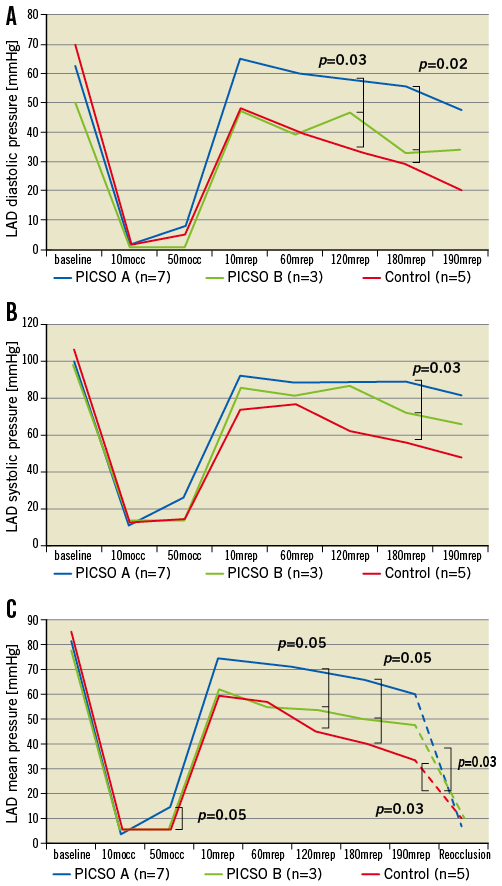
Figure 3. Distal left anterior descending coronary artery (LAD) pressure recordings across a pressure wire along the course of the experiments in the three study groups: A) diastolic pressure, B) systolic pressure, and C) mean pressure, with the diastolic flow slope (dashed lines) indicative of the forward driving pressure across the microvascular bed. A flat slope (small pressure difference) indicates poor forward flow (microvascular obstruction), while a steep slope (high pressure difference) indicates a good flow. mocc: minutes occlusion; mrep: minutes reperfusion
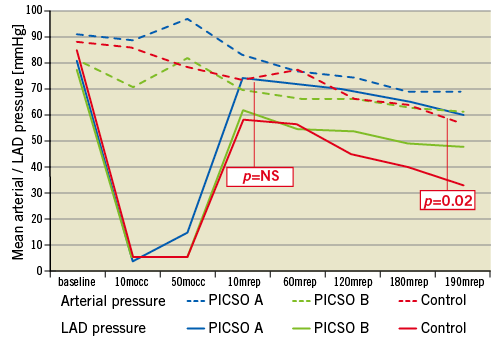
Figure 4. Distal left anterior descending coronary artery (LAD) pressure (mean pressure) plotted against mean systemic arterial blood pressure along the course of the experiments in the three study groups. The p-values indicated refer to the differences seen in group C. All differences in groups A and B were non-significant.
Soluble markers measured in coronary sinus blood are plotted in Table 2.
MYOCARDIAL AREA AT RISK AND NECROSIS
The AAR was comparable among groups. This was expressed as a percentage of the left ventricle as illustrated in Figure 5A. The infarct size was similar among groups and was generally very large (Figure 5B).
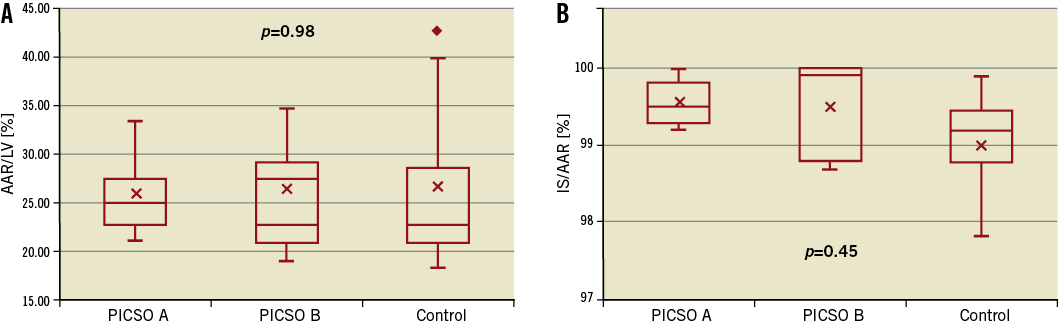
Figure 5. Area at risk as a percentage of the left ventricle, AAR/LV (A) and infarct size as a percentage of the AAR, IS/AAR (B).
IMMUNOFLUORESCENCE STAINING OF MYOCARDIAL TISSUE
Deposition of IgM was found in VIT and NIT of all groups and to a lesser extent in the non-ischaemic tissue (area not at risk, ANR, Figure 6A and Figure 6B). For C3c, no significant differences were found between ischaemic and non-ischaemic tissue in any of the groups (data not shown).
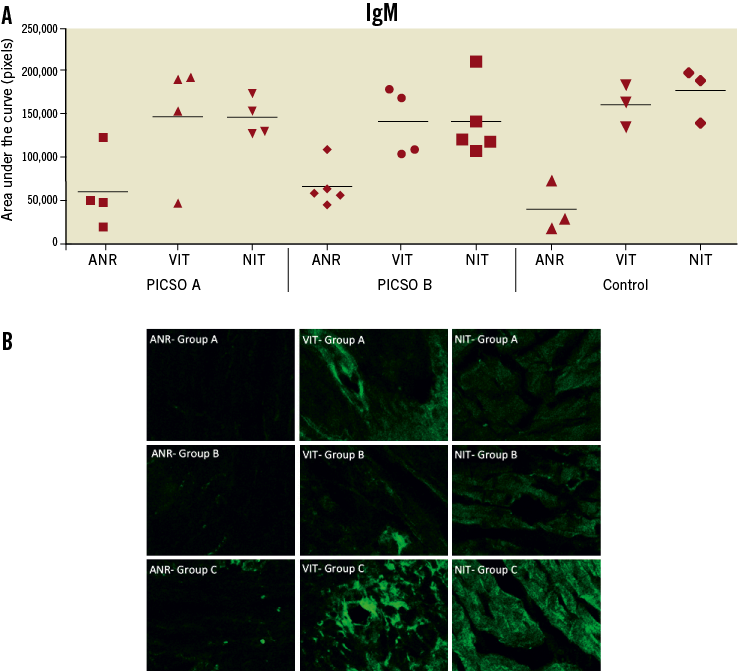
Figure 6. A) Quantitative evaluation of immunofluorescence staining data for natural IgM antibody deposition in myocardial tissue. B) Representative images of immunofluorescence staining for IgM. ANR: area not at ischaemic risk; VIT: viable ischaemic tissue; NIT: necrotic ischaemic tissue. A, B and C indicate the corresponding treatment group
H&E STAINING OF MYOCARDIAL TISSUE
Significantly more haemorrhagic lesions were seen in the NIT and VIT of group C compared to groups A and B (p=0.002; Figure 7A and Figure 7B). No significant differences were seen in tissue oedema and myocyte necrosis.
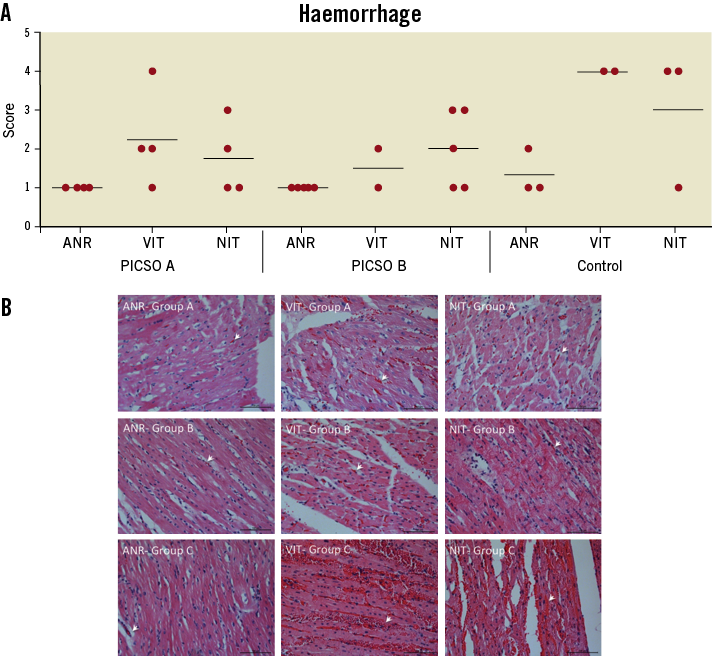
Figure 7. A) Haemorrhage scoring of H&E stained myocardial tissue and (B) representative images for H&E stains. A, B and C indicate the corresponding treatment group. ANR: area not at ischaemic risk; VIT: viable ischaemic tissue; NIT: necrotic ischaemic tissue
Discussion
The principal and novel finding of this study is that PICSO application in the setting of a closed-chest porcine myocardial ischaemia/reperfusion model significantly improved coronary perfusion pressure of the ischaemic myocardium consistent with reduced microvascular obstruction.
ICSO and PICSO result in a redistribution of coronary sinus blood flow within the venous compartment to the ischaemic myocardium through changes in pressure gradients throughout the coronary venous system9. The following mechanisms of anti-necrotic action were previously suggested: a functional venous microcirculation14, wash-out of reactive oxygen species, diminished granulocyte trapping, and improvement of cellular/interstitial oedema14,15. Importantly, it is very likely that the extent of coronary collaterals plays a crucial role in the efficacy of (P)ICSO in reducing infarct size, as evident in a series of experimental studies16-18. A meta-analysis of seven studies analysing the effect of ICSO in animal models revealed a significant reduction in infarct size of 29% in the treatment group compared with that in the control group (p<0.001; 95% CI: -40.9 to -17.7)19. It was previously shown in a canine ischaemia/reperfusion model that collateral flow to TTC-positive (viable-ischaemic) regions was significantly greater than collateral flow to TTC-negative (necrotic-ischaemic) regions (p=0.01), underlining the role of collaterals in preserving the viability of tissue and limiting infarct size in the ischaemic jeopardised myocardium3.
In patients with chronic stable angina ICSO/PICSO was able markedly to reduce ST-segment shifts on surface and intracardiac ECG occurring during a two to three-minute coronary artery balloon occlusion20,21. Furthermore, we showed that the amount of ischaemia reduction by ICSO is dependent on coronary collateral function. A minimal degree of collateral function appears necessary to render ICSO effective while it cannot manifest an effect when collateral function prevents ischaemia in the first place21.
Late mortality after acute myocardial infarction is attributed to two distinct aetiologies: chronic heart failure and/or malignant arrhythmias. While left ventricular systolic dysfunction is a determinant for developing heart failure22, and is mainly dependent on infarct size, electrical instability is not23. Markers indicative of electrical instability such as blunted heart rate turbulence and altered QT dynamicity are independent predictors of late mortality after acute myocardial infarction. Both were found to be directly related to the presence of myocardial microvascular obstruction after primary PCI largely independent of left ventricular function24,25. Our data demonstrate the ability of PICSO to improve coronary perfusion consistent with reduced myocardial microvascular obstruction as assessed by coronary pressure measurements which is an established tool in this respect12,13. Since left coronary blood flow is purely diastolic, the low distal coronary diastolic pressure expresses a diminished flow due to a maximally raised downstream resistance in the damaged microcirculation in the necrotic myocardium. According to Ohm’s law, coronary flow to the infarcted area must be reduced out of proportion relative to resistance, because a linear change of resistance and flow would result in a constant and preserved distal pressure. PICSO improved coronary flow consistent with reduced microvascular resistance as reflected by the preserved distal pressure26.
The non-significant difference in infarct size among groups and the overall large necrotic areas are an indication of the intrinsic limitation of the used animal model, namely its deficiency of collaterals. The very low LAD wedge pressures (about 5 mmHg in all groups) at 10 minutes ischaemia confirm the lack of arterial collateral flow. Retrograde perfusion with backwards transmission of pressure to the LAD as a proof of concept is seen at 50 minutes ischaemia in the significant rise of LAD wedge pressure in group A. Natural IgM antibodies are known to bind to neoepitopes expressed due to ischaemia and primarily trigger the classical and lectin pathways of complement activation, which contribute substantially to tissue injury27. Depositions of IgM were similar in all experimental groups (Figure 6A). However, only in the control group without PICSO treatment were the differences observed between the non-ischaemic ANR and vital (VIT) or necrotic (NIT) ischaemic myocardial tissue found to be statistically significant. Similar to IgM, no difference among groups was found for deposition of the complement component C3c. More haemorrhagic lesions were seen in the ischaemic myocardium of the control group. Intramyocardial haemorrhage in STEMI is associated with larger myocardial infarction and worse clinical outcome in humans28. We cannot reach a clear conclusion as to whether there was a difference in any of the aforementioned effects depending on the time point of PICSO application.
Other attempts to reduce reperfusion injury produced discrepant findings and included antioxidants, reduction of intracellular Ca2+ overload and Na+–H+ exchange inhibitors, anti-inflammatory agents, adenosine, metabolic modulation, magnesium, therapeutic hypothermia and, more recently, ischaemic postconditioning, atrial natriuretic peptide, protein kinase C–delta inhibitor, glucagon-like peptide 1, long-acting erythropoietin, mitochondrial PTP, atorvastatin, as summarised by Yellon and Hausenloy29. A more effective approach may be to target more than one mediator of reperfusion injury at a time.
Twelve patients with anterior infarction treated with ICSO + thrombolysis were compared with 12 patients treated with thrombolysis alone. Enzymatic infarct size, left ventricular wall motion and Tl-201 perfusion defect at follow-up in the ICSO group were slightly improved compared with controls30.
With contemporary primary PCI practice for acute myocardial infarction, including thrombus aspiration, stent implantation and aggressive antithrombotic/antiplatelet treatment, a considerable number of patients still experience impaired myocardial perfusion, in spite of a patent IRA. As a consequence, this long pursued strategy of ICSO/PICSO has recently been regaining attention, as an adjunctive therapy to improve outcome after acute myocardial infarction.
In conclusion, the PICSO study device was effective in improving coronary perfusion pressure in this closed-chest porcine ischaemia/reperfusion model, besides being safe and feasible in its application.
Acknowledgements
The authors are grateful to Olgica Beslac and Daniel Mettler, Experimental Surgery Institute, and Katja Matozan and Carmen Fleurkens, Department of Clinical Research, both University of Bern, for their assistance during the experiments.
Funding
Miracor Medical Systems GmbH, Vienna, Austria.
Conflict of interest statement
A. Khattab and G. Stone have provided consultancy services for Miracor. The other authors have no conflicts of interest to declare.

