Abstract
Aims: To discriminate early ST-segment elevation myocardial infarction (STEMI) presenters at a high probability of successful pre-hospital thrombolysis (PHT) using a simple nomogram based on independent predictors of complete ST resolution.
Methods and results: OPTIMAL was an observational, prospective study undertaken at 79 medical centres in France in patients with STEMI undergoing pre-hospital thrombolysis (PHT) within six hours of symptom onset and coronary angiography within six hours of thrombolysis. The baseline and pre-coronary angiography ECGs of 800 patients were analysed. The main outcome measure was ST segment resolution ≥70%. ST resolution was associated with a significant reduction in mortality (1.8% vs. 4.3%; p=0.05). After multivariable logistic regression analysis, five independent predictors of successful myocardial reperfusion were identified: ≤1 h between pain onset and thrombolysis (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.18–2.62); body mass index (BMI) <30 kg/m2 (OR 1.74, CI 1.12–2.72); current/previous smoking (OR 1.71, CI 1.21–2.43); non-anterior infarct territory (OR 1.75, CI 1.27–2.41); and maximum amplitude of ST elevation <3 mm (OR 1.83, CI 1.32–2.54). The C-statistic of the model was 0.71 (95% CI 0.67-0.74). Using these five independent factors, a simple nomogram was developed to assess the probability of ST resolution after PHT. This nomogram allows discrimination of probabilities ranging from 13 to 72%.
Conclusions: This simple nomogram can predict the probability of successful myocardial reperfusion after thrombolysis. This may be useful in the triage of STEMI presenters.
Introduction
European and American guidelines on the management of acute myocardial infarction strongly recommend immediate initiation of fibrinolytic therapy in patients presenting with ST-segment elevation myocardial infarction (STEMI) when primary percutaneous coronary intervention (PCI) cannot be performed quickly after first presentation at a medical centre1,2. In this context, pre-hospital thrombolysis (PHT) is safe and has been shown to yield superior results compared with in-hospital thrombolysis3,4.
In France, the French emergency medical system (Système d’Aide Médicale d’Urgence [SAMU]) has promoted the use of PHT through mobile intensive care units with a physician on board, which provided an opportunity to reduce dramatically the time between symptom onset and reperfusion. In patients who present within the first two to three hours after the onset of symptoms, PHT is associated with early and 1-year mortality rates at least similar to if not better than those of primary PCI5-8. However, thrombolysis fails to achieve early recanalisation of the occluded infarct-related artery in almost half of the patients treated. Therefore, early identification of patients who are likely to have unsuccessful thrombolysis would be advantageous: it would allow these patients to be triaged to a strategy of primary PCI, even if the delay related to this treatment is longer than currently recommended in guidelines. For patients treated with thrombolysis but who fail to recanalise, this approach would identify patients requiring urgent coronary angiography in order to perform rescue PCI.
Recently, the Orientation of Patients Treated for Myocardial Infarction After Lysis (OPTIMAL) study provided quantitative data for predicting early patency of the infarct artery in patients treated with PHT9. However, myocardial reperfusion (as determined by ST resolution) is a much stronger correlate of improved clinical outcomes after myocardial infarction than is epicardial coronary patency (as determined by Thrombolysis In Myocardial Infarction [TIMI] 3 flow)10,11. The aim of this analysis of the OPTIMAL study was to identify predictors of myocardial reperfusion (judged from ST-segment resolution) after PHT, and to develop a simple, quantitative nomogram for predicting the likelihood of myocardial reperfusion according to standard clinical and electrocardiographic characteristics available in the emergency setting.
Methods
Study design
The methods used in the OPTIMAL study have been described elsewhere12. Briefly, this was a multicentre, observational, prospective cohort study conducted in 79 hospitals in France between November 2004 and November 2005. Each centre had a pre-hospital mobile intensive care unit with a physician on board and a coronary care unit with 24-hour access to coronary angiography.
To be eligible for inclusion in the study, patients (≥18 years) had to present with STEMI determined from a 12-lead electrocardiogram (ECG), defined as ≥0.1 mV in at least two peripheral leads or ≥0.2 mV in at least two precordial leads; to have received PHT within six hours of pain onset; and to be scheduled for coronary angiography within six hours of receiving thrombolytic therapy. Patients were not eligible for inclusion if they had an acute coronary syndrome without elevation of the ST segment; if they did not have a coronary angiography planned within six hours of the start of thrombolytic therapy; or if they were enrolled in another clinical trial.
Assessment of myocardial perfusion (STresolution) and data collection
ECGs from 800 patients performed just before thrombolysis (first ECG) and just before coronary angiography (second ECG) were read in a blinded fashion at a core laboratory. ST-segment resolution was assessed using a standard 12-lead ECG. The lead with the greatest magnitude of ST deviation in the infarct territory (measured 20ms after the J point) was selected on the first ECG performed immediately before thrombolysis. When two leads had an identical degree of ST deviation, the first lead encountered (in the following sequence: I, II, III, aVL, aVF, V1-V6) was taken into account13. The percentage of ST resolution was then measured on the same lead on the ECG performed immediately before coronary angiography14. Resolution was considered complete when the percentage reduction was at least 70%.
Additional variables collected were: time between symptom onset and start of thrombolysis; chest pain intensity before thrombolysis; haemodynamic status (blood pressure, heart rate and Killip class); previous medical history; drugs administered together with PHT; and performance and timing of in-hospital PCI.
Data were recorded on standardised case report forms (CRFs) by emergency physicians and cardiologists. Procedures were implemented to ensure that the patient database guaranteed anonymity and patients received an information leaflet that outlined their right to access, correct or withhold data. The study protocol was reviewed by the French Society of Cardiology and the study was performed in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC, USA). Categorical data are summarised as percentages; continuous variables are presented as medians with interquartile ranges (IQR). Univariate comparisons were carried out using the χ2 test for qualitative variables. Student’s t-test (or a non-parametric Wilcoxon test when the distribution of the variable was not normal) was used to compare quantitative data.
Variables associated with ST resolution (at least 70%) with a p-value <0.20 in the univariate comparisons were used for backward multivariable logistic regression analysis to determine odds ratios (ORs) that were independently associated with ST resolution. The beta-coefficients of the final multivariable model were used to compute an equation giving the predicted probability of achieving at least 70% ST resolution for individual patients according to their clinical and electrocardiographic characteristics before thrombolysis administration. This equation was used to develop a nomogram for predicting the likelihood of ST resolution following PHT. ORs are reported with 95% confidence intervals (CIs). Other thresholds were used to assess ST-resolution (50 and 60% ST-resolution) in a sensitivity analysis. Results were very similar.
The performance of the risk prediction model was assessed by evaluating discrimination through the estimation of the C-statistic of the final model, and by evaluating calibration through the Hosmer-Lemeshow chi-square statistic15. We performed an internal validation of the model using a bootstrapping method. We performed 1,000 random bootstrap samplings with replacement. Each sample comprised 800 subjects (same size as the original data set). The model was applied to each bootstrap sample and the C-statistic was estimated each time. The mean difference between the C-statistic estimated from using the original data set and the C-statistic estimated from using each bootstrap sample was calculated. This mean difference is a measure of the degree of over optimism introduced in the validation of our model. Finally, the C-statistic could be corrected by subtracting the degree of over optimism from the original C-statistic15.
Results
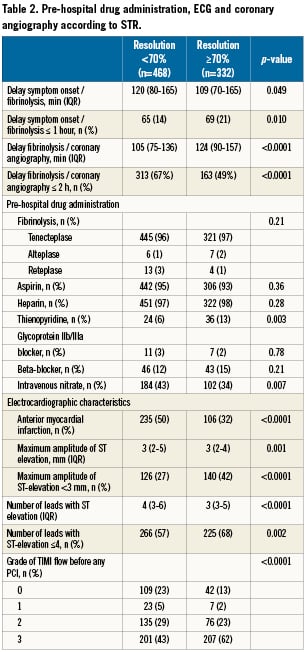
Among 1,159 patients with STEMI enrolled in OPTIMAL, a blinded assessment of ST resolution was possible, based on core laboratory data, in 800 patients (Figure 1). The patients’ clinical characteristics are summarised in Table1 and 2. PHT was performed a median of 115 min (interquartile range [IQR]: 75–165) after symptom onset, with 81% of patients receiving treatment within three hours. The median time from thrombolysis to coronary angiography was 110 min (IQR 80-147). In 96% of patients, the fibrinolytic drug administered was tenecteplase. After coronary angiography, PCI was performed in 90% of patients (47% rescue, 3% delayed and 50% despite TIMI 3 flow on coronary angiography), with a median of 1 (IQR 1-1) stent implanted (Table 2).
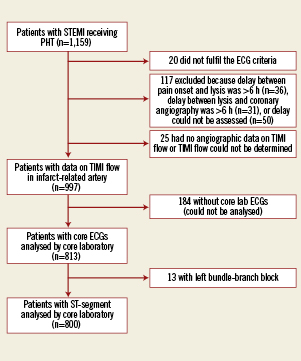
Figure 1. Patient disposition in the OPTIMAL study.
In-hospital clinical outcomes
In the population of 800 patients undergoing ST-resolution assessment, 51% (408/800) had a grade 3 TIMI flow in the infarct-related artery and 42% (332/800) achieved at least 70% ST resolution. Compared to patients with no or minimal ST-segment resolution, ST resolution ≥70% was significantly associated with better clinical outcomes in terms of death (P=0.05) and death or shock (P<0.01) (Figure 2).
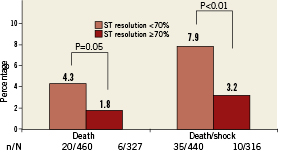
Figure 2. In-hospital outcomes according to myocardial reperfusion.
Univariate predictors of reperfusion
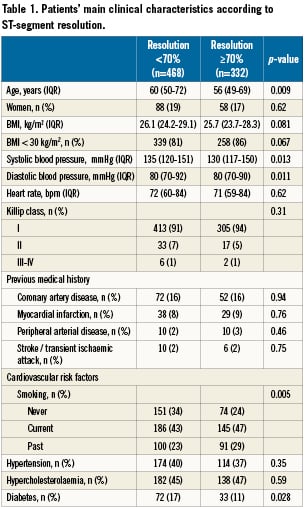
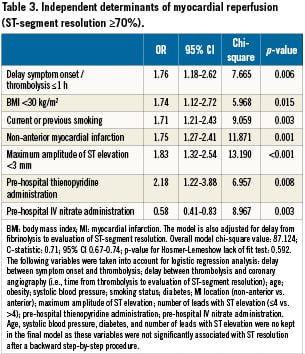
Among the data available in the pre-hospital setting, there were ten univariate correlates of ST-segment resolution (Table 1 and 2): younger age; lower blood pressure; short delay between symptom onset and thrombolysis and longer delay between thrombolysis and coronary angiography; current or past smoking; absence of diabetes; non-anterior infarct location (defined as lack of involvement of leads V1 to V4); smaller ST elevation; lower number of leads with ST elevation; treatment with a thienopyridine; lack of use of intravenous nitrates. A TIMI flow grade of 3 was also significantly associated with ST resolution.
Independent predictors of complete ST resolution
Using the data available before administration of PHT, five independent predictors of successful myocardial reperfusion (ST resolution ≥70%) were identified (Table 3): ≤1 hour between pain onset and receipt of thrombolysis; body mass index (BMI) <30 kg/m2; current or previous smoking; non-anterior infarct location; and maximum amplitude of ST elevation <3 mm. Since ST resolution was also independently associated with delay from fibrinolysis to evaluation of ST-segment resolution, and with thienopyridine and intravenous nitrate use during the pre-hospital management, the model presented in Table 3 was adjusted for these parameters.
Model performance
Our model had a good calibration (P-value for Hosmer-Lemeshow lack of fit test: 0.592), and a satisfying discrimination though it was not very high (C-statistic=0.71). The internal validation of the risk prediction model showed a degree of over optimism based on bootstrap simulation estimated at 0.019, leading to a corrected C-statistic at 0.69.
Nomogram for predicting ST-segment resolution
Based on these results, a simple nomogram was developed to help clinicians determine the probability of successful myocardial reperfusion after PHT (Figure 3). Each independent predicting factor accounted for 10 potential points, except the maximum amplitude of ST elevation <3 mm, which accounted for 11 points. Using the nomogram, the resulting scores predicting the probabilities of ST-segment resolution ranged from 13% to 72%.
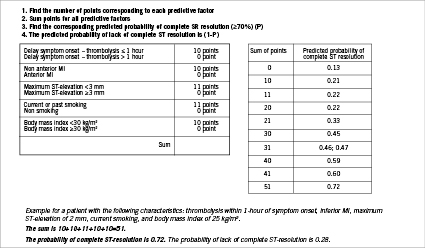
Figure 3. Nomogram for predicting myocardial reperfusion (≥70% ST-segment resolution).
Discussion
OPTIMAL is the largest study to date documenting early patency and ST-segment resolution following PHT. Study data identified delay between pain onset and receipt of thrombolysis, BMI, current/previous smoking, non-anterior infarct territory and maximum amplitude of ST elevation as five independent predictors of successful myocardial reperfusion. The simple-to-use nomogram, developed from these findings, may be valuable in the routine determination of the probability of successful myocardial reperfusion after thrombolysis and may enable improved treatment selection in STEMI presenters.
Thrombolysis is not always effective in ensuring rapid recanalisation of the infarct-related artery and may be less successful in achieving satisfactory reperfusion of the myocardium at risk. In the present study, a smaller proportion of patients had early ST resolution compared with patients who achieved grade 3 TIMI flow. This is supported by a previous study, which reported a higher percentage of patients with grade 3 TIMI flow than those with successful myocardial reperfusion after fibrinolysis plus angioplasty.16 Furthermore, myocardial contrast echocardiography demonstrated that many patients with apparently successful epicardial reperfusion do not have adequately restored coronary microcirculation (“no reflow”)17. Many other imaging techniques including cardiac magnetic resonance imaging18, positron emission tomography19, intracoronary Doppler flow velocity mapping20, and angiographic measures of microvascular “blush”10, have confirmed this inadequate microvascular perfusion.
Several reports in the literature support the concept that ST-segment resolution may be better correlated to prognosis and may be a better marker of successful thrombolysis than grade 3 TIMI flow in the infarct-related artery11,21. This is likely to be the result of ST-segment resolution improving both the patency of the infarct-related artery and blood flow in the microvasculature, and therefore offers additional information to that provided by measuring TIMI flow grade13. In addition, patients who regain normal epicardial flow following thrombolysis but show persistent ST-segment elevation are likely to have a poor prognosis13,22.
Although data from recent trials now consistently suggest the benefit of a systematic interventional approach after thrombolysis, the optimal timing for doing so is not clear23,24. The ASSENT 4 PCI study has rather suggested that it may be better to delay intervention by a few hours because of an increased risk of severe bleeding25. In that respect, the availability of our tool to help identify patients who are unlikely to have successful thrombolysis by any of these criteria could be clinically useful. This would allow early triage of patients receiving PHT to medical centres able to provide facilities for immediate angiography and, in cases of a persistently occluded infarct artery, emergency rescue PCI26. In addition, predicting success or failure of PHT would be useful in the selection of the appropriate reperfusion strategy when primary PCI is delayed substantially. In cases where PHT is likely to be successful, it could be given immediately. Conversely, in patients in whom it is unlikely to be successful, a longer primary PCI-related delay than is usually recommended may be acceptable.
Several randomised comparisons have shown that primary PCI is associated with improved outcomes compared with in-hospital thrombolysis27. However, the benefit of intravenous thrombolysis appears to be dependent on the time elapsed between symptom onset and initiation of treatment28. The results of a strategy of early initiation of PHT have been reported to be at least comparable to those of primary PCI in terms of early and 1-year mortality5,6. A subset analysis of the Comparison of Angioplasty and Pre-hospital Thrombolysis In acute Myocardial infarction (CAPTIM) study suggested that PHT may even be more effective than PCI in preventing mortality and cardiogenic shock in patients treated within two hours of symptom onset8, and that this benefit appears to be maintained over the long term29. In our study, a delay of ≤1 hour between symptom onset and receipt of thrombolysis was strongly associated with successful myocardial reperfusion, but the four other predictors were also important. The ‘beneficial’ effect of smoking on myocardial reperfusion outcome is well known and has been discussed in a previous study from OPTIMAL9. In patients with ST elevation ≥3 mm or with anterior STEMI, reduction in mortality may be the most important factor associated with early reperfusion, but PHT appears to be less effective. After primary PCI, patients with incomplete ST-segment recovery are also more likely to have an anterior infarct20,21. Feldman et al20 also reported that post-stent flow-velocity reserve in the infarct-related coronary artery is lower in anterior than in inferior infarcts, and the presence of an early systolic retrograde flow was found only in anterior infarcts. This finding suggests that microcirculatory function is more depressed in anterior infarcts versus non-anterior infarcts after reperfusion.
As seen in the present study, lower levels of cumulative ST-segment elevation observed at presentation were also associated with abortion of myocardial infarction, defined by Lamfers et al as ST resolution >50% and a rise of creatine kinase of less than twice the upper limit of normal30. The use of BMI as an independent prognostic factor after STEMI, when thrombolysis dose is adjusted according to weight, has received little attention.
Limitations
In contrast to previous patency studies, patients enrolled in OPTIMAL received contemporary treatments. In the present study, however, only a small proportion of patients received pre-hospital clopidogrel, since the results of COMMIT31 and CLARITY32 were not available when the study was designed and implemented. Another concern is the relatively large number of patients’ ECGs which could not be analysed by the core laboratory (n=184/997), although this reflects the technical issues with recording good quality ECGs in a pre-hospital setting in the context of acute STEMI. It is reassuring that the baseline characteristics of the patients excluded from core laboratory analysis are very close to those of patients analysed. The ability of OPTIMAL to discriminate the role of delays to lysis in association with ST-segment resolution is limited by the fact that the distribution of delays was quite narrow, with 75% of patients receiving thrombolysis 165 minutes or less after symptom onset. Finally, the model performs moderately well, with a C-statistic of 0.71 which may reflect that the variables collected may not fully characterise factors determining reperfusion.
Acknowledgements
We thank the doctors and nurses participating in the OPTIMAL study. Sophie Rushton-Smith, PhD, provided editorial services in the final version of this manuscript and was funded by Boehringer Ingelheim France.
Funding
Funding and sponsorship for the OPTIMAL study are provided by Boehringer-Ingelheim France. The sponsor had no involvement in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
A complete list of participating sites and SAMU/SMUR can be found in Bongard V, Puel J, Savary D, et al. Predictors of infarct artery patency after pre-hospital thrombolysis. The multicentre, prospective, observational OPTIMAL study. Heart 2008;0: hrt.2008.152504v1.
OPTIMAL scientific advisory committee
Emergency clinicians: F. Berthier (Nantes), J.-L. Bordonado (Bastia), S. Charpentier (Toulouse), P. Goldstein (Lille), Y. Lambert (Le Chenay), F. Lapostolle (Bobigny), A. Ricard-Hibon (Clichy), D. Savary (Annecy), J.-L. Sebbah (Gonesse), L. Soulat (Châteauroux), K. Tazarourte (Melun).
Cardiologists: L. Belle (Annecy), P. Coste (Bordeaux), Y. Cottin (Dijon), N. Danchin (Paris), K. Khalife’ (Metz), C. Loubeyre (Paris), J. Puel (Toulouse), F. Schiele (Besançon), P.-G. Steg (Paris), P. Virot (Limoges).
Conflict of interest statement
Funding and sponsorship for the OPTIMAL study are provided by Boehringer Ingelheim France. D. Miljkovic: employee of Boehringer-Ingelheim; G. Steg: research grant: Sanofi-Aventis (significant); speakers’ bureau (all modest): Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Nycomed, Sanofi-Aventis, Servier, The Medicines Company; Consulting/advisory board (all modest): Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Endotis, GlaxoSmithKline, Medtronic, Merck Sharp & Dohme, Nycomed, sanofi-aventis, Servier, The Medicines Company. Stockholding. The other authors have no conflict of interest to declare.

