Abstract
In remote, sparsely populated areas with long transfer distances to percutaneous coronary intervention (PCI) centres it is impossible to deliver PCI within the recommended time limits, and fibrinolysis should be the treatment of choice in patients with ST-elevation myocardial infarction (STEMI). Fibrinolysis should preferably be administered in the pre-hospital setting. Patients with contraindications to fibrinolysis, late presenters and patients with cardiogenic shock should be transferred for primary PCI, even when the transfer delays are substantial.
Fibrinolytic therapy is not the final step of reperfusion treatment, but should be followed by transfer to a PCI centre as soon as possible for rescue PCI or routine angiography with PCI if indicated. The optimal timing of routine angiography following fibrinolysis is not settled, but recent trials suggest a time window of two to 12 hours.
A well-organised system of care with clear treatment protocols and coordinated transfer systems is necessary for identifying treatment-eligible patients for on-site fibrinolysis or transfer for primary PCI, and for ensuring that therapies are available in a timely manner 24 hours a day, seven days a week. A well-organised STEMI network is also necessary for early transfer of lytic treated patients for rescue PCI or routine angiography.
Abbreviations
ASSENT-4: Assessment of the Safety and Efficacy of a New Treatment: Strategy with Percutaneous Coronary Intervention
CARESS-in-AMI: Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction
ECG: electrocardiogram
EMS: emergency medical system
GRACIA-1: Grup de Analisis de la Cardiopatia Isquemica Aguda
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
STREAM: The Strategic Reperfusion Early After Myocardial Infarction study
TIMI: thrombolysis in myocardial infarction
Introduction
Primary percutaneous coronary intervention (PCI) is the preferred reperfusion therapy in ST-elevation myocardial infarction (STEMI), as long as it can be delivered within 90-120 minutes from a patient’s first medical contact1,2. The percentage of STEMI patients treated with primary PCI is steadily increasing, and in most European countries primary PCI is the leading reperfusion strategy3. However, in areas remote from PCI facilities (e.g., mountains, islands, fjords), primary PCI cannot be delivered within the recommended time limits and less than ideal treatment options exist for STEMI patients. These include fibrinolytic therapy or transfer to a PCI hospital for primary PCI despite significant transfer delays. Recent European guidelines recommended fibrinolytic therapy as the treatment of choice if primary PCI cannot be performed by an experienced team within 120 minutes of first medical contact1,2. However, not all STEMI patients in remote areas should be treated with fibrinolysis: patients with contraindications to fibrinolysis and late presenters should be transferred for primary PCI, irrespective of the transfer delay. Studies also show that longer PCI-related delays may be acceptable in high-risk STEMI patients such as those with congestive heart failure and shock4-6. In this review, the optimal reperfusion method in areas remote from primary PCI centres will be discussed. Furthermore, the need for post-fibrinolysis angiography and PCI, and the importance of a well-organised STEMI network to offer optimal STEMI treatment in these areas are underscored.
Fibrinolysis versus primary PCI
Many studies have demonstrated the superiority of mechanical reperfusion over the pharmacological approach7-9. Even when patients had to be transferred from an initial institution to another, better clinical outcomes were achieved using coronary angioplasty compared to on-site fibrinolysis8,9. The generalisability of these findings to patients living in areas remote from PCI centres is however unclear, because transfer times were short in these randomised trials, but they are substantially longer in real life and in remote areas.
The benefit of fibrinolytic therapy is well established10. It is also well known that fibrinolytic treatment is more beneficial in patients presenting early after symptom onset. In a meta-analysis of 22 trials, a substantially larger mortality reduction was found in patients treated with fibrinolysis within the first two hours from symptom onset than in those treated later11. The earlier the patient is presented and the larger the area at risk at the presenting ECG, the more beneficial fibrinolytic therapy is. Contraindications for fibrinolytic therapy are listed in Table 1.
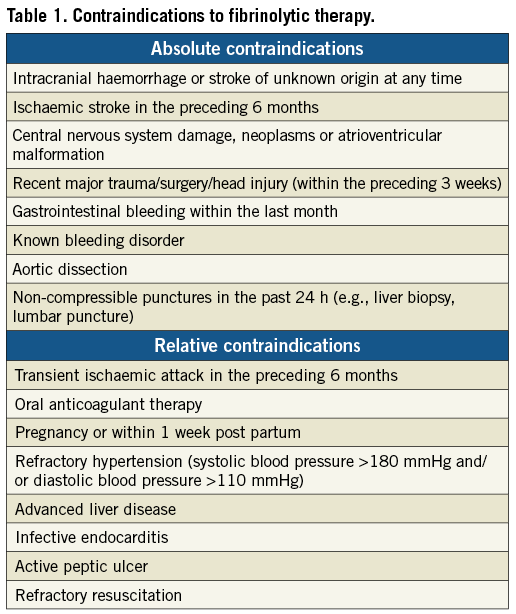
The beneficial effects of primary PCI are also time-dependent; mortality increases with increasing ischaemic time12,13. Furthermore, although primary PCI is commonly more effective than fibrinolytic therapy, the benefits of primary PCI compared with fibrinolysis decrease as the time delay for performing PCI increases. From randomised trials it has been calculated that a PCI-related delay of 80-120 minutes cancels out the survival benefit of primary PCI compared to fibrinolysis14,15. A recent analysis of the large NRMI register suggested that the beneficial effect of transfer for PCI is lost compared to on-site fibrinolysis, when the PCI-related delay is about 120 minutes16.
Evaluation of registry data has also shown that the acceptable PCI-related delay where the advantage of primary PCI over fibrinolysis is lost depends upon the characteristics of the patient16. This study indicated that this delay varied considerably according to age, symptom duration and infarct location: from <1 hour for an anterior infarction in a patient <65 years of age presenting <2 hours after symptom onset, to almost 3 hours for a non-anterior infarction in a patient >65 years of age presenting >2 hours after symptom onset. Recent data show that the acceptable PCI-related delay is also affected by the patient risk: in high-risk STEMI patients a longer PCI-related delay could be acceptable5,6. In patients with cardiogenic shock, primary PCI is the preferred treatment4.
This means that, to select the optimal reperfusion strategy for STEMI patients in remote areas, one should consider both patient characteristics and time delays. Patient age, duration of symptoms, infarct location, signs of heart failure, transport distance to PCI, transport facilities (ground ambulance or air ambulance available) and weather conditions should all be taken into consideration. Patient characteristics favouring on-site fibrinolysis vs. transfer for PCI are listed in Table 2. While the standard reperfusion strategy in Europe is primary PCI, fibrinolytic therapy is nevertheless the recommended choice for most patients living in areas remote from PCI facilities (Figure 1)1,2.
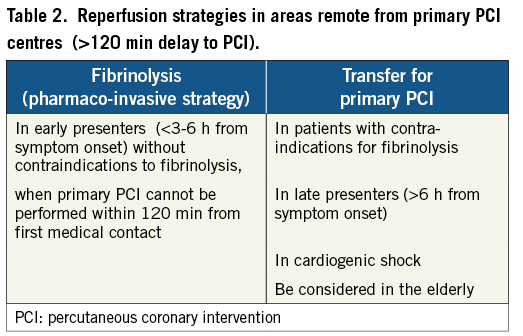
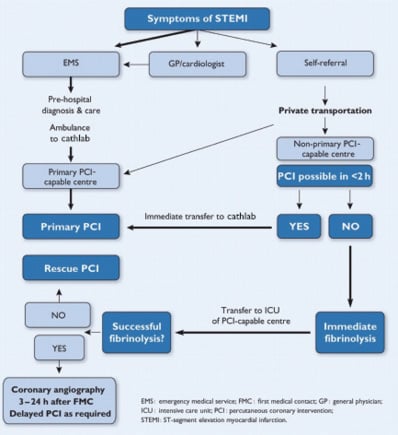
Figure 1. Recommended reperfusion strategies in ST-segment elevation myocardial infarction. Reproduced with permission from Wijns, Kolh, Danchin, et al2. PCI: percutaneous coronary intervention.
Pre-hospital administration of lytic therapy shortens time to treatment and yields better clinical outcomes than in-hospital administration17,18. If appropriate facilities exist, with trained medical or paramedical staff able to analyse on site or to transmit the ECG to the hospital for supervision, fibrinolytic therapy should be initiated in the pre-hospital setting. The aim is to start fibrinolytic therapy within 30 minutes of arrival of the ambulance. For patients arriving at a non-PCI hospital, a realistic aim is to initiate fibrinolysis within 30 minutes (door-to-needle time).
A fibrin specific agent should be preferred: recombinant t-PA (tissue plasminogen activator; alteplase) or mutants of t-PA such as r-PA (reteplase) or TNK-tPA (tenecteplase)18. Tenecteplase can be given as a single bolus facilitating more rapid treatment in and out of hospital. Aspirin, clopidogrel and enoxaparin are recommended as antithrombotic co-therapy with fibrinolysis (for doses, see reference 1).
The need for angiography and PCI following fibrinolysis
Fibrinolysis fails to achieve TIMI 3 flow in the infarct-related artery in 30%-50% of cases. In cases of failed fibrinolysis, patients should undergo immediate angiography and rescue PCI if indicated19. To offer patients angiography as soon as possible in cases of failed fibrinolysis, all patients should be transferred to a PCI centre as soon as possible following initiation of lytic therapy.
Even if it is likely that fibrinolysis was successful, a strategy of routine early angiography is recommended if there are no contraindications1,2. Several randomised trials have shown that early routine angiography following fibrinolysis, with subsequent PCI if required, reduced the rates of reinfarction and recurrent ischaemia as compared with a “wait and watch” strategy, in which angiography and revascularisation was indicated only in patients with spontaneous or induced severe ischaemia or left ventricular dysfunction20-24 (Figure 2). The benefits of early routine angioplasty after fibrinolysis were seen in the absence of increased risk of adverse events (stroke or major bleeding). Thus, early referral for angiography with subsequent PCI if indicated is recommended routinely following thrombolysis. Accordingly, optimal reperfusion in areas remote from primary PCI centres is a “pharmaco-invasive strategy” (a combination of a pharmacological and a mechanical approach).
The optimal time window from lysis to angiography is not settled. Time varied from a median of 1.6 hours in the CAPITAL-AMI study to 16.7 hours in the GRACIA-1 study (Figure 2)18,24. As suggested by the ASSENT-4 study25, very early angio/PCI following fibrinolysis might increase the risk of ischaemic complications. However, the three most recent trials all had a median time delay between lysis and angiography of two to three hours, and showed improved outcomes with no increased risk of bleeding and no thrombotic complications compared to later angiography20-22. Trials included in the meta-analysis by Desch et al24 had a delay between fibrinolytic administration and angiography in the range of 1.1-16.7 hours and >70% of the patients were included in trials with a delay to angiography of <3 hours. There were no apparent safety differences in the trials relative to the time to PCI in the given time range. The more intense antithrombotic co-therapy used in recent fibrinolysis trials as well as increased use of the radial approach may have contributed to these results. The current ESC revascularisation guidelines recommend performing routine angiography between three and 24 hours following fibrinolysis (Figure 1)2, but a shorter time window of two to 12 hours may be advocated based on recent trials.
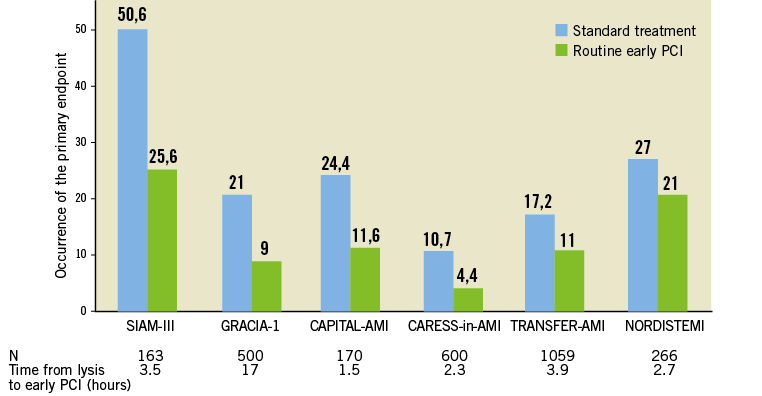
Figure 2. Studies comparing early routine percutaneous coronary intervention (PCI) versus standard therapy after fibrinolytic therapy in ST-elevation myocardial infarction. Reproduced with permission from Halvorsen and Huber18.
The pharmaco-invasive strategy has improved outcomes significantly for patients treated with fibrinolysis. A couple of randomised trials as well as data from French registries have reported outcome data for pre-hospital fibrinolysis followed by early angiography comparable to those of primary PCI26,27. However, as these randomised trials were small, more evidence is needed. The on-going Strategic Reperfusion Early After Myocardial Infarction (STREAM) study evaluates a strategy of primary PCI compared to pre-hospital lysis followed by invasive evaluation in early presenting STEMI patients with long (>60 minutes) transfer delays to PCI28. This trial will add valuable information on the optimal treatment strategy for STEMI patients living in remote areas without PCI facilities.
The importance of STEMI systems of care
To deliver optimal reperfusion treatment within the recommended time limits to all STEMI patients, it is recommended to build up and organise systems of care (STEMI networks) in which emergency medical systems (EMS), non-PCI capable hospitals and hospitals with PCI facilities co-operate closely29-31.
Such systems of care are especially important in areas remote from PCI centres. Optimisation of systems of care in these areas includes clear regional treatment protocols, ambulances equipped with ECG and mobile telemetry units for ECG transfer for pre-hospital diagnosis and triage, training of ambulance personnel in basic and advanced cardiac life support as well as in administration of pre-hospital thrombolysis, and organising coordinated regional transport systems. STEMI networks can diagnose and treat patients in a pre-hospital situation, guide transport to a PCI-capable hospital, activate the catheterisation laboratory and the personnel at the time of pre-hospital diagnosis, making it possible to bypass both non-PCI hospitals and the emergency room in the PCI centre, and reduce inter-hospital transfer delays. Integrated STEMI networks have been shown to minimise time delays to treatment, and have also led to a significant reduction of in-hospital mortality29.
In Minnesota, a standardised PCI-based treatment system for STEMI patients from 31 hospitals up to 210 miles from the PCI centre in Minneapolis was developed in 200331. A standardised protocol was implemented at each hospital, depending on the distance to the PCI centre. Patients who presented to hospitals <60 miles away were transferred for primary PCI. Patients who presented to hospitals >60 miles away received half-dose fibrinolytic therapy followed by emergency transfer for immediate PCI (pharmaco-invasive strategy). Transferred patients were taken directly to the cardiac catheterisation laboratory for PCI without re-evaluation in the emergency department. Outcomes for STEMI patients transferred from remote hospitals utilising a pharmaco-invasive strategy were compared to those who presented to a PCI centre directly, and there were no significant differences in 30-day mortality (5.5% vs. 5.6%; p=0.94), stroke, major bleeding or re-infarction between patients receiving a pharmaco-invasive strategy and patients presenting directly to the PCI centre, despite a significantly longer door-to-balloon time31. Their experience demonstrated that treatment of STEMI patients in areas remote from a PCI centre may be effective and safe using a standardised protocol with a pharmaco-invasive reperfusion strategy and an integrated transfer system.
In south-eastern Norway, a similar protocol was set up in 2004. In this region, which is large with rural and mountain areas in the north and west, there was one high-volume PCI centre located in the city of Oslo (Oslo University Hospital Ullevål) and there were eight non-PCI hospitals (Figure 3). More than half of the patients in this region live in the city of Oslo including its suburban areas, and had short transfer times to the PCI centre. However, the transfer distance to PCI for patients living in the northern part of the region was up to 400 km. All the ambulances in the region were equipped with ECG and mobile telemetry units for ECG transfer to the community hospitals or the PCI centre, and the paramedics in the ambulances >100 km north of Oslo were trained to deliver fibrinolysis in the ambulance with clinical decision support provided by the coronary units at the community hospitals. A helicopter ambulance with a physician on board was located in the northern part of the area. Patients presenting to an ambulance <100 km away from the PCI centre were transferred directly to the PCI centre for primary PCI. Patients presenting to an ambulance or a community hospital >100 km away from the PCI centre were given fibrinolytic treatment with tenecteplase, preferably in the pre-hospital setting, if time from symptom onset was <6 hours and there were no contraindications. In case of late presentation or contraindications for fibrinolysis, patients were transferred for primary PCI irrespective of the transfer delay. All lytic treated patients were transferred to the PCI centre in Oslo for rescue PCI or routine angiography within the first 24 hours. PCI-treated patients were usually returned to the community hospitals the following day.
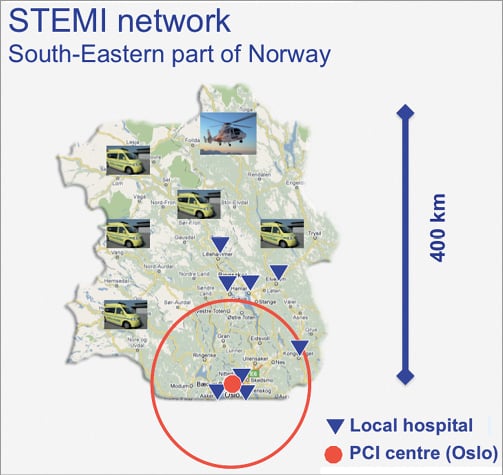
Figure 3. Map of the south-eastern part of Norway with the PCI centre in Oslo, Oslo University Hospital Ullevål (red bullet), the non-PCI hospitals (blue triangles) and the ambulances. The red circle shows the area with <100 km transfer distance to the PCI centre.
In such well-organised STEMI networks, it is possible to offer primary PCI within the recommended time to most patients and pre-hospital fibrinolysis followed by immediate transfer to a PCI centre to patients living in remote areas. Clear regional treatment protocols, appropriately equipped ambulances or helicopters, trained paramedic staff and regional coordination of transport systems make reperfusion therapy of most STEMI patients achievable within recommended timeframes and with low mortality29-31. The local environment, the economic and the clinical realities must be considered when creation of regional protocols and adoption of pharmaco-invasive strategies are considered.
Conclusion
In remote, sparsely populated areas with long transfer distances to PCI it is impossible to deliver primary PCI within the recommended time limits, and fibrinolysis should be the treatment of choice in early presenting STEMI patients without contraindications. However, fibrinolysis is not the final step of reperfusion treatment. Following fibrinolysis, patients should be transferred to a PCI centre as soon as possible for rescue PCI or routine coronary angiography with PCI if indicated. This pharmaco-invasive strategy has improved outcomes significantly for patients treated with fibrinolysis. Several studies have reported outcome data for this pharmaco-invasive strategy comparable to those of primary PCI. In patients with contraindications to fibrinolysis and in late presenters, transport for primary PCI should be arranged irrespective of transfer delays. To deliver optimal reperfusion treatment within the recommended time limits to all STEMI patients, it is recommended to build up STEMI networks. In well-organised STEMI networks, it is possible to offer primary PCI within the recommended time to the majority of patients, and pre-hospital fibrinolysis followed by immediate transfer to a PCI centre to patients living in remote areas.
Conflict of interest statement
S. Halvorsen has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Sanofi-Aventis.

