The Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial was a multicentre, prospective, randomised, controlled, open-label trial comparing manual thrombus aspiration followed by PCI to PCI only in patients with ST-elevation myocardial infarction (STEMI)1. Patients were enrolled from the national Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and endpoints evaluated through national registries. The primary endpoint was all-cause mortality at 30 days, and 7,244 patients with STEMI presenting within 24 hours of onset of symptoms were recruited. TASTE found no significant difference in 30-day mortality (2.8% in the aspiration group versus 3.0% in the no aspiration group, hazard ratio 0.94, 95% CI: 0.72-1.22). Rates of hospitalisation for recurrent myocardial infarction at 30 days were 0.5% and 0.9% (p=0.09), respectively. Rates for stent thrombosis were 0.2% and 0.5 % (p=0.09). There were no significant differences in stroke rates (Figure 1).
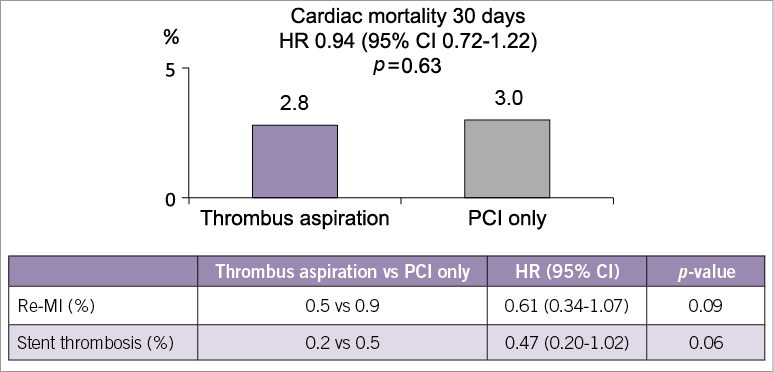
Figure 1. Summary slide taken from PCR trials book.
The chair (Andreas Baumbach) opened the session by polling the audience to ask if their attitude to thrombus aspiration had changed since the publication of TASTE. More than half the audience felt that their use of aspiration had decreased as a result.
Michael Haude presented an angiogram of an inferior STEMI in a typical patient. The actual treatment given to the patient was going to be revealed at the end of the session.
Gennaro Sardella reviewed what was known about thrombus aspiration before TASTE. Improved myocardial blush score, cardiac mortality and reduced non-fatal myocardial infarction at one year were seen with manual aspiration in the TAPAS trial2. In the most recent meta-analysis of 25 trials of manual aspiration, it was associated with a 24% relative risk reduction of MACE and a 29% relative risk reduction in mortality at a median follow-up of six months3. Currently, the 2013 ACCF/AHA STEMI guidelines state that manual thrombus aspiration is reasonable for patients undergoing primary PCI (Class IIa, Level of Evidence B)4.
Alexandra Lansky took us through her thoughts on TASTE. In her view the trial design was innovative: recruiting via a population-based registry facilitated patient enrolment and data collection. Recruitment through a registry has several advantages: large numbers are generated, it is cost-effective, takes all-comers, follow-up is complete and recruitment is quick. However, because there was no independent event adjudication, stent thrombosis and myocardial infraction reporting may be unreliable. For the patient population, 7,244 of the eligible 11,709 STEMI patients were randomised, questioning the generalisability for the patient population. Patients were not randomised for a variety of reasons, including no informed consent (38%), and the operator deciding that aspiration was needed (7%). The population of patients who underwent randomisation did have significant differences from those who did not (less diabetes, younger, less previous CABG), and is therefore not entirely generalisable to all STEMI patients. Furthermore, the outcomes of randomised patients were significantly better than of those who did not undergo randomisation (a 30-day mortality rate of 2.9% versus 10.6%).
Mortality at 30 days was the primary endpoint of the trial, which may present several limitations. The original power calculation suggested that a total planned enrolment of 5,000 patients (based on a one-month control mortality of 6.3% and a 30% reduction with thrombus aspiration at 30 days) would be required. When enrolment approached 5,000, the 30-day mortality was observed to be lower than expected (2.9%) in the study cohort, so the sample size had to be enlarged, now assuming a 50% reduction in 30-day mortality. A 30-day endpoint is probably too early to demonstrate a significant difference (prior trials and meta-analyses demonstrating a mortality benefit from thrombectomy showed that it was not apparent before six months; in TAPAS the mortality benefit was at one year). In addition, a 50% reduction in mortality seems ambitious (few therapies in medicine ever show such a mortality benefit). With these considerations in mind and essentially an underpowered trial for the chosen endpoint, the negative mortality finding in TASTE may not mean that thrombectomy is without clinical value. Both myocardial infarction and stent thrombosis were numerically reduced at 30 days, providing a signal of benefit with thrombectomy. Importantly, there was no increased stroke with aspiration and the procedure was safe. Ultimately, the one-year follow-up will be a critical measure to determine whether thrombectomy has a meaningful impact on mortality or other secondary endpoints.
Sanjit Jolly discussed the design of the TOTAL trial, a randomised trial of routine aspiration ThrOmbecTomy with PCI versus PCI ALone in patients with STEMI undergoing primary PCI5. This randomised controlled trial aims to enrol 10,700 patients and has a composite of cardiovascular death, recurrent MI, cardiogenic shock, or new or worsening NYHA Class IV heart failure at six months.
Michael Haude then presented the conclusion to his case demonstrating successful use of thrombus aspiration. He stated that, if aspiration is performed correctly and carefully, it is a very useful technique whose benefits include removal of vasoactive substances in addition to thrombus. He felt that, in an occluded vessel in STEMI, aspiration is better than clot disruption with a balloon.
At the end of the session, Andreas Baumbach re-polled the audience, and this time the majority feeling was that further trial data (including TOTAL and the one-year results of TASTE) are required prior to recommending changes in clinical practice.
Conflict of interest statement
A. Baumbach has received Speakers Fees from Astra Zeneca. The other authors have no conflicts of interest to declare.

