
Abstract
Aims: Simple surface modifications can enhance coronary stent performance. Ultra-hydrophilic surface (UHS) treatment of contemporary bare metal stents (BMS) was assessed in vivo to verify whether such stents can provide long-term efficacy comparable to second-generation drug-eluting stents (DES) while promoting healing comparably to BMS.
Methods and results: UHS-treated BMS, untreated BMS and corresponding DES were tested for three commercial platforms. A thirty-day and a 90-day porcine coronary model were used to characterise late tissue response. Three-day porcine coronary and seven-day rabbit iliac models were used for early healing assessment. In porcine coronary arteries, hydrophilic treatment reduced intimal hyperplasia relative to the BMS and corresponding DES platforms (1.5-fold to threefold reduction in 30-day angiographic and histological stenosis; p<0.04). Endothelialisation was similar on UHS-treated BMS and untreated BMS, both in swine and rabbit models, and lower on DES. Elevation in thrombotic indices was infrequent (never observed with UHS, rare with BMS, most often with DES), but, when present, correlated with reduced endothelialisation (p<0.01).
Conclusions: Ultra-hydrophilic surface treatment of contemporary stents conferred good healing while moderating neointimal and thrombotic responses. Such surfaces may offer safe alternatives to DES, particularly when rapid healing and short dual antiplatelet therapy (DAPT) are crucial.
Abbreviations
BMS: bare metal stent
CoCr: cobalt-chromium alloy
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
LL: late loss (luminal)
QCA: quantitative coronary angiography
SE: standard error
UHS: ultra-hydrophilic surface
Introduction
Next-generation drug-eluting stents (DES) reduce in-stent restenosis and stent thrombosis risks when accompanied by appropriate dual antiplatelet therapy (DAPT)1. Still, concern persists over toxicities of locally eluted drug and inflammation induced by polymer coatings2,3. It remains important to consider whether evolution in stent design can improve performance, potentially obviating the need for drug/polymer coatings.
Surface wettability alters thrombotic and inflammatory interactions. In vitro evidence suggests that hydrophilicity reduces platelet adhesion and improves endothelialisation4-6, yet contemporary stents are mildly hydrophilic at best (Figure 1A)4. Though phosphorylcholine-coated stent surfaces are considered “hydrophilic”, a water contact angle of ~85° indicates their amphiphilic nature4,7 (90° is typically considered the transition between hydrophobic and hydrophilic surfaces). Many factors restrict DES polymer hydrophilicity, such as swelling of hydrophilic polymers in aqueous environments, too rapid drug release in the absence of hydrophobic constituents, and emerging concerns over polymeric embolisation3,5,8,9.
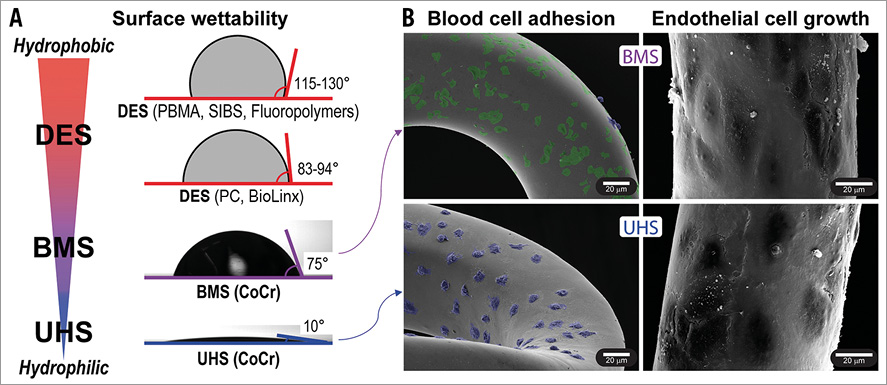
Figure 1. In vitro impact of stent ultra-hydrophilic surface treatment on cellular response. A) Water contact angles for common DES polymers4 and bare metal CoCr, without (BMS) or with ultra-hydrophilic treatment (UHS). B) In vitro response of BMS and UHS stents (SEM images). Left: stents after 2 hrs incubation with heparinised human blood, showing platelet adhesion (green) on BMS and neutrophil adhesion (blue) on UHS stents (images artificially coloured for improved cell visibility). Right: after 72 hrs of endothelial cell proliferation, showing confluent cell coverage on both BMS and UHS stents.
We here consider an ultra-hydrophilic surface (UHS) treatment that activates metallic surfaces6, achieving a contact angle of 10°. This is far less than commercial DES polymers or stainless steel and cobalt-chromium (CoCr) bare metal surfaces (Figure 1A). When compared with untreated bare metal, UHS show promising results in vitro, dictated by alterations in surface chemistry and native oxide layer which tune blood protein absorption and cell adhesion (Figure 1B)6,10. This study extends the implications of ultra-hydrophilicity in vivo and in the context of next-generation stents. FDA-approved second-generation DES and bare metal stents (BMS) were compared with matched UHS-treated stents in A) porcine coronary models to assess intimal hyperplasia and late thrombosis; and B) a rabbit iliac model to assess early healing and subacute thrombosis (Figure 2).

Figure 2. Study design and baseline characteristics. A) Study flow chart. Based on in vitro outcome of UHS and BMS platforms, in vivo studies were designed and performed to evaluate the response of UHS versus contemporary BMS and DES. Evaluation of late tissue response (30, 90 days) compared UHS to both DES and BMS; early healing (≤1 week) focused on UHS/BMS comparisons. Porcine coronary and rabbit iliac models were employed. B) Number of implanted stents and baseline characteristics (porcine and rabbit models) for the Integrity BMS, Resolute DES, treated-Integrity UHS main analysis.
Methods
STENT PLATFORMS AND HYDROPHILIC TREATMENT
We compared contemporary DES and BMS with UHS-treated stents. Our main analyses considered the Integrity BMS and the Resolute Integrity® DES (3.0×18 mm platforms; Medtronic, Minneapolis, MN, USA). Pooled analyses using additional DES and BMS (XIENCE PRIME® vs. MULTI-LINK 8™; Abbott Vascular, Santa Clara, CA, USA; PROMUS™ vs. Omega™; Boston Scientific, Marlborough, MA, USA) assessed consistency. For reporting, UHS, DES, and BMS labels reference the main treated-Integrity/Resolute/Integrity comparisons, respectively, unless indicated.
All UHS were prepared by surface treatment of corresponding BMS6. The treatment reduces nickel and cobalt concentrations in the surface oxide and simultaneously removes organic contamination, leading to ultra-hydrophilic surfaces. The water contact angle on CoCr discs fell from 75°±5° to 10°±5° after treatment (Figure 1).
IN VITRO UHS CHARACTERISATION
The effect of ultra-hydrophilic treatment applied to stents was first evaluated in vitro using methods recently applied for metallic discs6. Briefly, treated and untreated Integrity stents were rinsed with 0.9% NaCl solution and incubated statically with 2 ml of whole, 3 IU/ml heparinised human blood (ethics committee approved). After two hours at 37°C, samples were rinsed, paraformaldehyde-fixed, and dried (graded ethanol series; critical-point dryer) for scanning electron microscopy (SEM) (Hitachi S-3400N-II; Hitachi, Tokyo, Japan) analysis. Adherent cells were classified based on shape and fluorescent staining of analogous samples from the same batches.
Human aortic endothelial cell proliferation assays were performed using protocols detailed elsewhere6. Cells were cultured on CoCr Integrity stents (treated and untreated) and grown under standard culture conditions (humidified atmosphere, 5% CO2, 37°C) in an endothelial growth medium (EGM-2) with full supplements (Lonza) and 10% foetal bovine serum. Endothelial cell proliferation on stents after three days was evaluated by SEM following fixation and drying protocols used in the blood incubation study.
ANIMAL MODELS
Animal studies (porcine coronary and rabbit iliac) (Figure 2) were performed at CBSET (Lexington, MA, USA; AAALAC accredited) in accordance with federal USDA and institutional IACUC oversight (IACUC porcine protocol #I00041; rabbit protocol #I00037).
PORCINE CORONARY MODEL
Nineteen Yorkshire swine (three, 30-day time points) and seven Yucatan mini swine (90-day) were used to evaluate stent responses with baseline and follow-up angiography, and post-necropsy histology. The Yucatan model was used to assess long-term response to limit the excessive growth of Yorkshire pigs1. Animals underwent intervention on day 0. In each animal, up to four stents were implanted into coronary arteries via carotid access. Animals were anticoagulated with intravenous heparin (procedural activated clotting times >275 s). DAPT consisted of oral aspirin and clopidogrel (loading dose: 650 mg and 300 mg, respectively, the day prior to implantation; 81 mg and 75 mg daily thereafter).
Quantitative coronary angiography (QCA) (Centricity Cardiology CA1000 Cardiac Review 2.0; GE Healthcare, Little Chalfont, United Kingdom) was performed on day 0 (baseline, on balloon inflation, and following deployment), and prior to euthanasia (day 3, 30, or 90). QCA data (LL, percent stenosis) are reported as group mean±SE.
Baseline artery diameters and balloon-to-artery ratios were equivalent across stent types and time points (all values within 2.8±0.17 mm and 1.14±0.05, respectively; mean±standard deviation).
At the specified endpoints and following angiography, animals were euthanised under maintenance anaesthesia (inhaled isofluorane; 0.1%-5.0%) via overdose of potassium chloride (1-2 mmol/kg IV; rapid bolus). Following euthanisation, hearts were perfusion fixed in 10% formalin. Stented arteries were assessed using histomorphometry. Continuous morphometric data (percent area stenosis and neointimal thickness, mm) are presented as mean±SE; ordinal data (inflammation, neointimal fibrin, endothelialisation, platelet adhesion scores) were taken as average per stent and reported as median and range (median [low high]).
RABBIT ILIAC MODEL
To evaluate early healing further, we used a rabbit iliac model permitting stent implantation in straight segments with minimal variability11. Overlapping stents (Integrity 3.0×18 mm) were used to accentuate re-endothelialisation differences12. Six New Zealand White rabbits underwent intervention (day 0) with right and left iliac artery stent implantation (12 UHS, 12 BMS; n=6 overlapped regions flanked by single stent zones). Stents were deployed using fluoroscopy. Animals were anticoagulated with heparin (1,000 U IV); antiplatelet therapy (aspirin, 40 mg) was administered one day prior to intervention and daily thereafter. On day seven, animals were euthanised. Stented arteries were processed and assessed using SEM. Olympus MicroSuite™ Biological Suite, version 2.5 (Olympus Corporation of the Americas, Center Valley, PA, USA) was used for morphometry and scoring (endothelial coverage and confluence, strut coverage, thrombus)13. Ordinal scores were taken as average per stent and reported as median and range (median [low high]).
STATISTICAL ANALYSIS
Statistical tests were performed in SigmaPlot 11.0 (Systat Software, Inc., San José, CA, USA). The two-sample Mann-Whitney test was used for ordinal comparisons (one-sample where indicated, when comparisons were made to a saturating threshold). The Shapiro-Wilk test for normality was performed on continuous data. When normal, continuous comparisons were performed with the unpaired Student’s t-test assuming unequal variances; comparisons to thresholds were made using the one-sample t-test; when not normal, the Mann-Whitney test was used (p-values <0.05 were considered significant). Hierarchical, pooled analyses were conducted using a linear mixed effects model.
Results
IN VITRO BLOOD INTERACTIONS WITH ULTRA-HYDROPHILIC BMS (UHS)
Characterisation of UHS vs. BMS Integrity stents with SEM showed a strong reduction in platelet adhesion with increased nucleated cell (neutrophil) adhesion (Figure 1B). This finding agrees qualitatively with prior quantitative tests on equivalent surfaces under static and dynamic incubation conditions6,10. Endothelial proliferation was rapid on both UHS and BMS surfaces, with confluent cell coverage on all stents after three days (Figure 1B).
UHS REDUCE LATE INTIMAL HYPERPLASIA COMPARED WITH DES IN VIVO
Baseline porcine deployments (artery diameter and overstretch ratio) were comparable (p>0.1). Stent thrombosis never occurred and no areas of malapposition were observed. QCA demonstrated 2.7-fold or greater reduction in % stenosis and LL at 30 days (p<0.025) and at 90 days in UHS vs. DES (p=0.016) (Figure 3A). Histology corroborated angiography, demonstrating significant reductions in histological % stenosis and neointimal thickness on day 30 (p<0.048) (Figure 3B, Figure 3C). Similar 30-day trends in QCA and histology were observed with XIENCE PRIME and PROMUS DES versus platform-matched UHS (n=3 to 4). Pooled histology data demonstrated reduced inflammatory scores (pMann-Whitney=0.044), injury (pMann-Whitney=0.001), neointimal thickness (p<0.001) and stenosis (p<0.001)) (Figure 3D) for pooled UHS as compared to pooled DES.
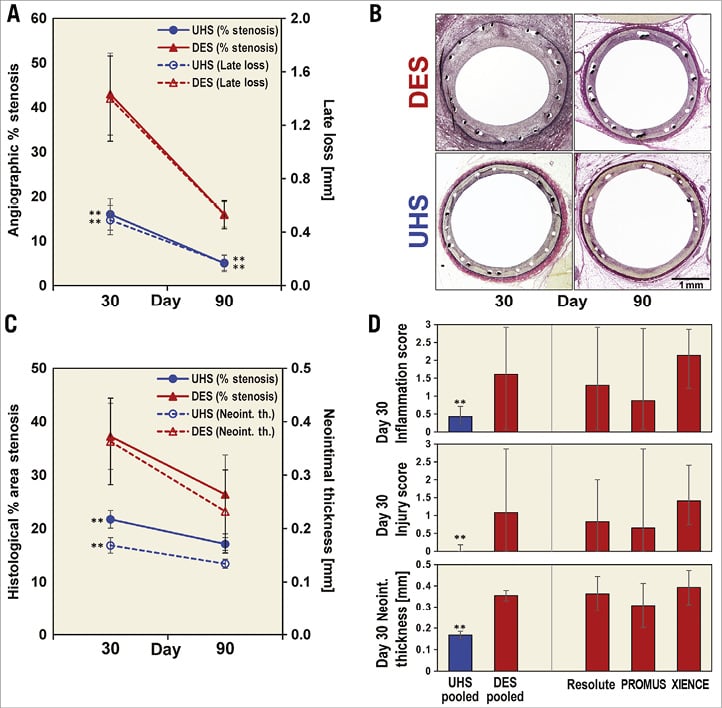
Figure 3. Swine model results. A) QCA: lower % stenosis and LL in UHS compared with DES (treated-Integrity vs. Resolute). B) Representative haematoxylin and eosin staining for UHS and DES at 30 and 90 days (treated-Integrity and Resolute). C) Histology quantification: lower % area stenosis and neointimal thickness in UHS compared with DES (treated-Integrity vs. Resolute). D) Sensitivity analysis for pooled UHS (n=16) and DES (n=14) 30-day histological comparisons supporting decreased stenotic response in UHS: inflammation score (medians±25/75% quartiles), injury score (medians±25/75% quartiles), neointimal thickness (mean±SE). Injury score was independent from balloon:artery overstretch ratio, which was comparable for all samples. **denotes p<0.05 in panels A, C, D.
UHS HAVE FAVOURABLE TIME COURSE RESPONSES COMPARED TO BMS IN VIVO
We examined porcine responses to UHS and BMS at three, 30 and 90 days (Figure 4). Day-three QCA did not demonstrate significant differences in % stenosis or LL between UHS and BMS (Figure 4A). By day 30, both measures increased across stent type, less so in UHS, which demonstrated lower % stenosis and LL than BMS (p=0.01 and 0.008, respectively) (Figure 4A). A non-significant trend to reduced % stenosis for UHS compared to BMS persisted at day 90 (p=0.16) (Figure 4A). Histomorphometry corroborated QCA (Figure 4B, Figure 4C). Day-three histology results were similar between UHS and BMS (Figure 4C). By day 30, UHS resulted in an ~1.5-fold reduction in both neointimal thickness and % stenosis (p=0.011 and 0.013, respectively) that faded to non-significant trends by day 90 (Figure 4C). Pooled day-30 UHS-BMS analyses confirmed consistency (UHS-treated MULTI-LINK 8 and UHS-treated Omega versus respective BMS, n=3 to 4 each) (Figure 4D), showing reduced neointimal thickness, inflammation, injury and % stenosis (single platform differences not statistically significant due to low sample number).
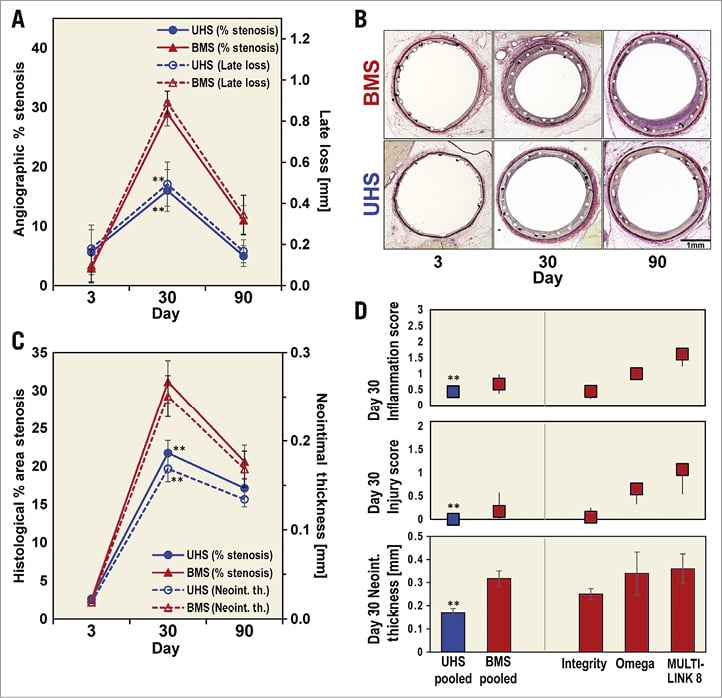
Figure 4. Swine model results. A) Three, 30, 90-day time course for QCA. After initial, similar 3-day responses in % stenosis and LL in UHS and BMS (treated-Integrity vs. Integrity), responses diverged with a peak difference at 30 days, showing significantly reduced % stenosis and LL in UHS. B) Representative haematoxylin and eosin staining for UHS and BMS (treated-Integrity vs. Integrity). C) Histology quantification corroborating QCA findings. D) Sensitivity analysis for pooled UHS and BMS 30-day histological comparisons, supporting decreased stenosis in UHS: inflammation score (medians±25/75% quartiles), injury score (medians±25/75% quartiles), neointimal thickness (mean±SE). Injury score was independent from balloon:artery overstretch ratio, which was comparable for all samples. **denotes p<0.05 in panels A, C, D.
DELAYED DES HEALING TRACKS WITH THROMBOTIC INDICES COMPARED WITH RAPID HEALING IN BMS AND UHS
The endothelialisation rate was similar in UHS and BMS (Table 1), confirming in vitro observations (Figure 1B). One out of four BMS samples showed an endothelialisation score <3 combined with some histological platelet adhesion after three days, which may indicate a less favourable short-term response compared to UHS, but this is not statistically significant due to the low sample size.
By day 30, both UHS and BMS platforms endothelialised fully and platelet adhesion was absent. In contrast, a trend to delayed endothelialisation was observed in DES at day 30 (again, due to low sample size this is not statistically significant, pMann-Whitney=0.10). Adherent platelets were observed in the DES samples with subconfluent endothelialisation. Complete endothelialisation without platelets was observed across platforms by day 90.
DES showed elevated fibrin scores at day 30 compared to both UHS and BMS (pMann-Whitney≤0.005), which was no longer significant by day 90 (Table 1). In a mixed effects analysis across pooled samples from the main study (UHS, BMS, and DES; n=51), reduced endothelialisation correlated with platelet adhesion (slope=–1.3 [–1.08 –1.52], p<1e-5) and fibrin (slope=–1.1 [–0.61 –1.65], p=5.7e-5). Note, pooling was performed by centring around median values for each time point.

In the overlapping rabbit iliac model, endothelialisation and thrombus scores of UHS and BMS were similar by day seven (Figure 5A, Figure 5B). A single BMS outlier (median-centred pGrubb’s=0.007) was detected: this stent exhibited both the lowest endothelialisation and highest thrombus score (red values in Figure 5B). When porcine and rabbit data were pooled, such suboptimal healing cases were present for both BMS and DES, though not observed in UHS (Figure 5C).
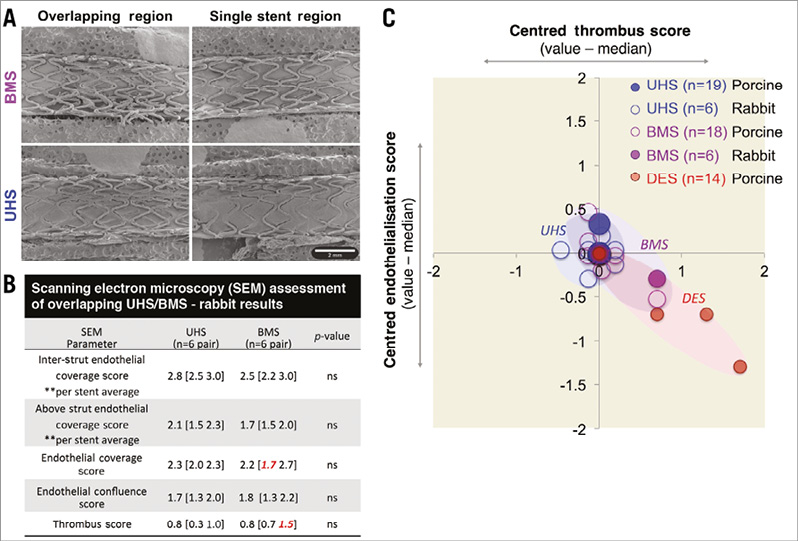
Figure 5. Rabbit model results and overall association between reduced endothelialisation and increased indices of thrombosis. A) SEM images of rabbit iliac arteries, 7-day post-overlap stenting with UHS (lower panel) and BMS (upper panel). B) Endothelial coverage (inter-strut, above strut, overall; 0-4), endothelial confluence (0-3), and thrombus (0-4) scores demonstrating similar early healing patterns between UHS and BMS (treated-Integrity vs. Integrity). In a total of 12 cases (six UHS, six BMS), a single outlying BMS case was noted with reduced endothelial coverage and increased thrombus score; pGrubb’s=0.007). C) When normalised endothelial coverage and thrombosis metrics were pooled for both rabbit and porcine models, suboptimal healing and thrombosis correlated (R2=0.78) and occurred in 8% of cases (two BMS, three DES), none of which was UHS.
Discussion
Next-generation DES improve safety and efficacy1, yet risks in specific populations and issues surrounding cost-effectiveness continue to pose challenges5,14. We demonstrate the in vivo potential of non-eluting, non-polymeric, ultra-hydrophilic stent surfaces in promoting early healing while limiting hyperplasia and thrombosis.
UHS AND IN VIVO RESPONSE
UHS healing and intimal responses were similar or better than second-generation DES or contemporary BMS. UHS performed uniformly in terms of inflammation, injury and neointimal hyperplasia, regardless of animal or stent platform, indicating consistent effect. DES platforms delayed re-endothelialisation and were associated with increased inflammation, injury and fibrin (Figure 3, Table 1) compared to both UHS and BMS. These results are in line with literature data15 and prompted assessment of short-term healing of UHS against BMS (Figure 2). The results, both in swine and rabbit models, indicate that endothelialisation of UHS occurred at least as fast as for BMS, confirming in vitro findings.
Moreover, the animal models demonstrated that UHS reduced neointimal hyperplasia compared to tested DES and BMS (Figure 3, Figure 4). Reductions in % stenosis, LL and neointimal thickness were most significant at 30 days. Neointimal reduction of UHS compared to DES exceeded expectations. The high neointimal hyperplasia, inflammation, and injury scores for DES tracked together and were a result of high inter-sample variance. This was probably a consequence of known inflammatory reactions in pig coronaries to DES drug polymers4,15-18. Injury scores did not correlate with overstretch ratio (equivalent for all samples) in UHS, BMS and DES of otherwise identical platform design and implantation characteristics. This suggests that injury (as assessed through elastic lamina disruption) may be driven by inflammation. The decrease of neointimal hyperplasia among all devices at 90 days compared to their respective values at 30 days is in line with the literature15,19,20 and generally led to statistically non-significant differences among the stents at 90 days. It should be acknowledged that this longitudinal progression may be in part attributable to the different swine strains used for short- and long-term assessment.
We observed increased neutrophil adhesion to hydrophilic surfaces in vitro (Figure 1B), similar to a study of glass (contact angle: 13°) versus Teflon (108°) that observed a >2 fold increase in hydrophilic-related neutrophil adhesion21. While some studies implicate neutrophils in atherosclerosis and vascular response to balloon injury, others suggest a more complex process with benefit in promoting healing22-24. Given the ongoing debate, such in vitro results are best taken cautiously and require in vivo consideration in applicable conditions. Though our animal models also demonstrated that UHS reduced inflammation and hyperplasia while promoting early healing, these must be substantiated by evaluation in patients and diseased vessels.
Indeed, despite the superior efficacy of DES in reducing hyperplasia in clinical studies1, this need not be the case in the healthy porcine artery model, where equivalence with BMS is often observed18,25. This can in part be attributed to the high level of overstretch injury that is induced by stent expansion within diseased human vessels (often >100%), where the relative DES performance may be enhanced, as compared to low overstretch levels of 10-20% (15% in this study) while stenting healthy porcine arteries1,25. On the other hand, the good performance of UHS in the healthy porcine model suggests that it may be particularly efficient in low-overstretch situations, such as in neurovascular aneurysm treatment. For such indications, the low thrombogenic UHS surface property might also be beneficial.
Our findings also corroborated the relationship between delayed healing and clotting. A correlation was found between incomplete endothelialisation and measures of thrombosis (platelet adhesion; thrombus score). Even though these events were rare, the fact that outliers combining worst healing and thrombogenic scores never occurred with UHS suggests the robust performance of UHS in protecting against thrombosis (Table 1, Figure 5). Again, these results are in line with in vitro observations (Figure 1B) which showed markedly reduced platelet adhesion on UHS stents compared to BMS after blood incubation. This effect is attributed to shifts in chemical and surface charge properties of the metallic surface after UHS treatment, steering surface protein adsorption following blood contact6,10,26.
EVOLVING RISKS AND BENEFITS OF STENTING AND CO-ADMINISTERED DAPT
Second-generation DES improve safety and efficacy1, yet risks in specific populations, concerns over polymer/drug biocompatibility and issues surrounding cost-effectiveness continue to pose challenges and have motivated the search for polymer-free and non-drug-eluting platforms5,14,27. With concerns over delayed DES healing, clinical practice shifted to prolonged, ≥12 month DAPT following DES implantation to offset stent thrombosis risk5,14,28. When early DAPT cessation is likely, BMS followed by one month DAPT is an option, though in-stent restenosis must be considered. Various strategies have been developed to limit the excessive restenosis observed with BMS, such as surface modification with layers of pyrolytic carbon, silicon carbide, diamond-like carbon or addition of a passivating oxide layer29. While most strategies have failed to provide a clinically meaningful advantage with respect to restenosis, use of a titanium-nitride-oxide (TiNOX) layer has demonstrated competitive potential in head-to-head comparisons with contemporary DES30. By promoting healing comparable to BMS while mitigating restenosis, ultra-hydrophilic modifications might offer an alternative strategy, particularly in patients at high bleeding risk or where DAPT adherence is a concern31. Such issues are heightened in non-industrialised countries where access to next-generation devices and supportive DAPT is limited32.
Conclusions
In the context of contemporary, commercial stent platforms, we demonstrate that ultra-hydrophilic surface treatment may provide a safe, cost-effective means to quell restenotic and thrombotic risks in the absence of drug, while optimising early healing. This hypothesis is currently being considered through clinical evaluation (NCT02176265, http://clinicaltrials.gov/). Primary endpoints are in-stent LL and neointimal thickness at six months with one month of DAPT.
| Impact on daily practice In vivo studies of ultra-hydrophilic treatment of stent surfaces showed high potential of low neointimal hyperplasia and reduced thrombogenicity in healthy porcine and rabbit models. An ongoing clinical study seeks to confirm these promising results in the clinical praxis. |
Funding
This work was supported by unrestricted research sponsorship from Qvanteq AG. The Commission for Technology and Innovation (CTI) provided financial support for in vitro investigations. E.R. Edelman and K. Kolandaivelu received partial support from the National Institutes of Health [R01GM049039] and American Heart Association [12FTF12080241].
Conflict of interest statement
A. Zucker and S. Buzzi are employed by Qvanteq AG. D. Bhatt has received research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, and The Medicines Company, and has participated in unfunded research for FlowCo, PLx Pharma, and Takeda. The other authors have no conflicts of interest to declare.

