Abstract
Aims: The aim of this study was to determine the role of potential triggers of stent thrombosis.
Methods and results: Patients (n =437) with “definite” ST were recruited consecutively in the setting of a large multicentre observational cohort study. Patients were interviewed with validated questionnaires to identify one of the following triggers: i) timing of onset of ST, ii) performance of vigorous (≥ 6 MET) physical activity in the two hours preceding ST, iii) presence of emotional stress (experiencing a serious life event in the 14 days preceding the ST or feelings of anger in the 12 hours of ST) and iv) presence of a documented active infection at the time of ST. A total of 363 patients (83.1%) were able to supply adequate information. A significant trigger was identified in 83 patients (22.9%). Analysis of the different categories according to timing of ST revealed a higher prevalence of triggers with an increasing time-interval between index PCI and ST. Analysis of circadian variation showed a steep peak incidence from 7am-12pm.
Conclusions: Triggering mechanisms such as time of the day, physical exertion, emotional stress and infection may play an important role in a considerable number of patients presenting with ST, in particular in patients with (very) late ST.
Abbreviations
ARC: American Research Council
CRP: C-reactive protein
DSTR: Dutch Stent Thrombosis Registry
LAD: Left artery descending
MET: Metabolic equivalent
PCI: Percutaneous coronary intervention
SRRS: Social Readjustment Rating Scale
ST: Stent thrombosis
Introduction
Stent thrombosis (ST) is a feared complication of percutaneous coronary intervention (PCI) because it is associated with considerable morbidity and mortality.1-4 Given the devastating clinical consequences of ST, comprehensive risk stratification to identify those patients at high risk for this catastrophic event is mandatory. Previous studies have identified several important clinical, procedural and angiographic predictors that are associated with ST. These include acute coronary syndromes as the indication for PCI, premature discontinuation of clopidogrel therapy, high on-treatment (clopidogrel) platelet reactivity, bifurcation stenting, diabetes mellitus, renal failure, LAD stenting, impaired left ventricle ejection fraction, small stent diameter and long total stent length.2-9
Nonetheless, it is surprising why only a small subgroup of patients with risk factors for ST will eventually develop ST. Or the other way around, there remains a group of patients experiencing ST that is not characterised by the above mentioned conventional determinants. Consequently, further identification of superimposing mechanisms beyond the currently known risk factors will probably advance our understanding of the pathogenesis of ST.
Although several triggering mechanisms of myocardial infarction have been well established, only anecdotal evidence exists on the association between triggers and stent thrombosis.10-12 Given a certain degree of similarity in most pathophysiological pathways between ST and myocardial infarction8, we sought to extrapolate the triggering factors that are commonly known for myocardial infarction (such as timing of onset13-15, vigorous physical exercise15-19, infection20-23 and emotional stress24-28) to the arena of stent thrombosis.
Methods
The present study is a sub study of the Dutch Stent Thrombosis Registry (DSTR)1,7. In brief, the DSTR is a large-scale, multi-centre study conducted in three high-volume centres (>2,500 interventions per centre per year) in The Netherlands. All consecutive patients with an angiographically confirmed stent thrombosis (“definite” according to the ARC-criteria29) between January 2004 and February 2007 were enrolled. Stent thrombosis was categorised according to the timing of the event: acute (occurrence within the first 24 h after the index-procedure), subacute (from 24 h to 30 days), late (from 30 days to one year) and very late (>1 year after the index procedure).
Patient interview
All patients enrolled in the DSTR were intensively interviewed using standardised questionnaires about the conditions and activities in the time frame preceding the stent thrombosis. To minimise bias in ascertainment, conditions and circumstances elicited from patients in response to the open question: “Please describe in detail what you were doing in the hours before the onset of chest pain” were recorded. The open question was followed by a set of predefined, well-validated questions about the hypothesised triggers. The interviewers used a structured data abstraction and questionnaire form for data acquisition.
Timing of onset
The onset of discomfort was used as the onset time for ST. This reported time was checked with PCI-reports. All acute ST’s were excluded from this analysis, to eliminate the influence of PCI-time on the circadian variation.
Physical exercise
Patients were asked whether they had performed any physical activity in the two hours preceding the stent thrombosis. The degree of physical activity intensity was quantified by the Compendium of Physical Activities30, a coding scheme that classifies physical activity by rate of energy expenditure. This list, developed to enhance comparability of results across studies using self-reports of physical activity, characterises specific physical activities (both daily activities and sports) based on the standard of a metabolic equivalent (MET)16,30. The MET is used to estimate the amount of oxygen used during physical activity. One MET correlates with the energy (oxygen) required sitting down quietly. Any activity that burns three to five METs is considered moderate-intensity physical activity. Activity that burns ≥ 6 METs is considered vigorous-intensity physical activity. Patients were considered to have been engaged in vigorous exertion if they reported a peak MET of six or more in the two hours preceding the ST.
Infection
Patients were asked about any signs and conditions indicating the presence of an infection at the time of ST. All medical records were checked and laboratory charts were screened for inflammatory and infectious parameters indicative for an infection, including positive cultures, antibiotics use, (hs)-C-Reactive Protein (CRP), blood sedimentation rate (BSE), leukocyte count and leukocyte differentiation. Referring hospitals, general practitioners and pharmacies were also contacted to obtain additional information. Only documented infections (confirmed in clinical records) or by means of at least one positive cultures in combination with a C-reactive protein level >100 (mg/L).
Emotional stress
To study the impact of emotional stress as a potential trigger, two components of emotional stress were considered relevant: 1) life events and 2) anger.
LIFE EVENTS
To objectify the impact of life events, the Social Readjustment Rating Scale (SRRS) by Holmes and Rahe was used31,32. This scale has been designed to assess the cumulative stress of several positive or negative life events, as measured over the last year. The SRRS consists of a list of 43 life events. These items are ranked in order from the most impact (death of spouse, 100 points) to the least impact (minor violations of the law, 11 points). The number of “Life Change Units” that apply to events in the past year of an individual’s life are added and the final score will give a rough estimate of how stress affects health.
Because the aim of this study was to determine whether a life event can provoke stent thrombosis, the SRRS was slightly modified. Instead of using the cumulative incidence of life events in the last year, the occurrence of life events in the two weeks prior to the stent thrombosis was recorded. Furthermore, as in our opinion the clinical relevance of the life events is rapidly decreasing towards the bottom of the list, only life events mentioned on the upper half of the SRRS were regarded as potential triggers (i.e., the first 22 of 43 items, corresponding to ≥29 points).
ACUTE EMOTIONAL STRESS
Beside life events, other acute emotions (e.g., anger and extreme anxiety) can also induce stress. Given the subjective character of this category of mental stress, only episodes of anger were recorded as an potential mental stressor, as this emotional trigger has been most intensively investigated24,33. The requirement for this acute emotional stressor was the occurrence of anger within 12 hours preceding the stent thrombosis.
Statistical analysis
Continuous variables were reported as medians with 25th and 75th percentiles, and categorical variables were reported as frequencies with percentages. The chi squared test was used to compare categorical variables and for trend in proportions. A p-value <0.05 was considered significant. Descriptive statistics was performed with SAS, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
The number of triggers determined was presented as “number of triggers identified” for the several separate of triggers and as “number of patients in whom a trigger was identified” for the cumulative number of triggers.
Results
A total of 437 patients were enrolled in the DSTR. Of these, 56 (12.8%) patients died before the interview had taken place. Cardiovascular causes (including stent thrombosis) accounted for 88.8% of all deaths. Fourteen (3.2%) patients were lost-to-follow-up and four (0.9%) patients were not able to supply adequate information. These patients were also excluded from the analysis (Figure 1).
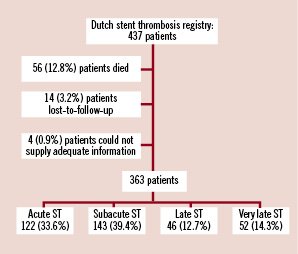
Figure 1. Study design and subject disposition.
The remaining 363 patients (83.1%) were able to supply adequate information. In the majority of cases, data were collected by direct patient interview. In eight cases (2.2%) the partner instead of the patient was interviewed because of communication problems (e.g., language problem, previous history of cerebrovascular accident). The median time between the ST and patient interview was 11 months (25th-75th percentiles: 6-18 months).
Timing of onset
The hourly distribution of the timing of the onset of chest pain is depicted in Figure 2.
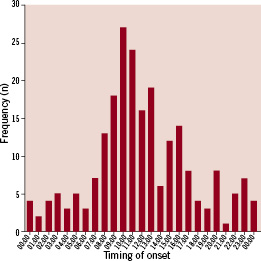
Figure 2. The hourly distribution of the timing of the onset of the chest pain.
A marked circadian variation – although less pronounced in patients with late or very late ST – in frequency of symptom-onset was observed with a minimum of events during night hours and a steep increase in events in the morning hours from 07:00 to 12:00 (noon). This six hour time-interval accounted for ~50% of all ST.
Triggering factors
Eighty-three patients (22.9%) reported the presence of at least one triggering event or condition. Four patients reported two different triggers. In two patients both an infection trigger and an emotional trigger were recorded, whereas in two other patients both an infection trigger and a physical exercise trigger was recorded. Figure 3 shows the prevalence of the different triggers preceding the stent thrombosis subdivided in categories according to the timing of ST.
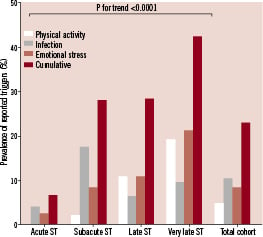
Figure 3. The prevalence of the different triggers preceding the stent thrombosis subdivided in categories according to the timing of ST. Separate triggers displayed as percentage identified triggers; cumulative charts displayed as percentage patients with an identified trigger.
The cumulative prevalence of the different triggers in the acute group is fairly low, whereas the prevalence of triggers in the subacute, late and very late group is higher. Analysis of the different categories of ST revealed a higher prevalence of triggers with an increasing time-interval between index PCI and ST (p for trend <0.0001).
Physical exercise
A total of 28 patients reported that they had performed physical exercise preceding the onset of ST. Of these, in 10 patients the MET was <6 and these patients did not fulfil the requirements for a significant exercise trigger. In the remaining 18 patients (5.0%), vigorous physical exertion (MET ≥6 ) was identified as a trigger preceding ST.
Infection
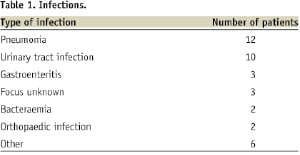
Thirty-eight patients (10.5%) reported the presence of an infection on the day of the stent thrombosis. Review of medical charts, laboratory parameters and cultures confirmed the presence of an active infection in all cases. The different types of infections are summarised in Table 1.
Emotional stress
A total of 31 patients (8.5%) reported the occurrence of a life event or feelings of anger preceding the stent thrombosis. Twenty-two patients (6.1%) experienced a life event within two weeks prior to the stent thrombosis. According to the SRRS, the mean ± SD score was 52±17 points, ranging from a minimum score of 29 (corresponding with “change of responsibilities at work”) to a maximum score of 100 (corresponding with “death of spouse”).
Nine patients (2.5%) reported an episode of anger within 12 hours preceding the ST.
Risk factors
The prevalence of risk factors is generally comparable in patients with and without a trigger preceding the stent thrombosis, although patients in whom a trigger was identified are on average three years younger (Table 2).
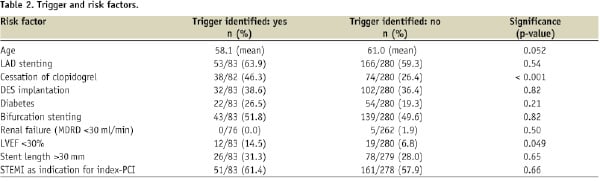
In addition, left ventricular ejection fraction <30% and cessation of clopidogrel at the time of stent thrombosis were more frequently observed in patients with a trigger.
Discussion
The present study is the first exploratory study investigating the relationship between triggering mechanisms and the occurrence of stent thrombosis in a large consecutive cohort of patients with ST, although no causal conclusions could be drawn from these descriptive data.
Substantial experimental and clinical evidence from the 80s and 90s strongly supports a causal relationship between several triggering mechanisms (such as timing of onset, strenuous exercise, presence of an infection and emotional stress) and the occurrence of myocardial infarction. Given the fact that ST and myocardial infarction share some, but not all, pathophysiological mechanisms, it is likely that these triggers may play a role in the pathophysiology of ST as well. However, only anecdotal evidence exists on the role of triggering mechanisms in ST.10-12
Despite the absence of a plaque rupture in the initial cascade of events leading to ST, numerous studies have revealed that other relevant physiologic processes are stimulated by several triggering mechanisms and contribute to the formation of an occlusive thrombus in the implanted coronary stent. These processes include increased sympathetic activity and vagal withdrawal, elevation in plasma catecholamines and renin levels, increased thrombin generation, increased heart rate and blood pressure, exercise induced coronary-artery spasm, increased systemic inflammation, increased vascular resistance, increased vessel-wall stress, a heightened platelet reactivity status and a hypercoagulability state34-38. These effects are mediated by complex mechanisms, involving α2-adrenergic receptor expression, von Willebrand factor platelet interaction, GPIIb/IIIa interaction, P-selectin expression of platelets and the release of nitric oxide.
With regard to physical exercise, several studies revealed paradoxical effects of moderate exercise and vigorous exercise on platelet function.39,40, suggesting that moderate exercise suppresses platelet reactivity and increases fibrinolysis. Conversely, vigorous exercise – especially in untrained individuals – enhances both platelet reactivity and coagulation, whereas it promotes fibrinolysis as well. From this perspective, moderate-intensity activity could be considered safe, whereas vigorous exercise might lead to a prothrombotic state ultimately leading to the formation of a thrombus.
In the present study, a surprisingly high percentage (almost 25%) of patients with ST reported a trigger. Analysis of the categories of ST revealed a higher prevalence of triggers with an increasing time-interval between index PCI and ST. Interestingly, the prevalence of the studied triggering mechanisms was the highest (42%) in the group of patients presenting with a very late stent thrombosis.
The lowest prevalence of the studied triggering mechanisms was found in patients presenting with acute ST. This observation is in line with previous findings identifying mechanical and procedural factors as the predominant cause of acute stent thrombosis41,42. Consequently, the pathophysiology of acute stent thrombosis should be considered distinct from the other categories of stent thromboses.
The identification of potential triggering mechanisms of ST might have important clinical implications related to both prognosis and prevention26. From a prognostic perspective, the presence of in particular emotional stress may imply that certain individuals are more vulnerable to stress-induced biological responses than others35. Consequently, the presence of emotional stress should be considered as a marker of increased risk. In addition, previous studies found personality to be an important predictor of adverse clinical outcome: type-D personality (patients high in negative affectivity and social inhibition) was independently associated with myocardial infarction and death in patients undergoing PCI with stent implantation.43 Of even more importance, Denollet et al demonstrated that the interaction effect of negative emotions with social inhibition – more than negative emotions alone – is associated with major adverse cardiac events. These findings might provide additional clues to identification of specific patients at increased risk of stent thrombosis.44
In relation to prevention, the ideal approach should involve a range of various strategies for the different types of triggering mechanisms. First, patients undergoing coronary stent implantation should be encouraged to perform moderate physical activity on a regular basis, because the beneficial effects of exercise training in the secondary prevention of coronary artery disease have been well established.45 In addition, several epidemiological studies demonstrated that the performance of moderate exercise on a regular basis lowers both the baseline risk as well as the relative risk that an episode of heavy physical exertion will trigger myocardial infarction16,19,46. However, caution remains warranted when patients plan to perform vigorous exercise, especially when untrained. According to the guidelines of the ACC/AHA47, patients should undergo an exercise test under supervised conditions, before starting to perform vigorous exercise.
Second, it has been known for several decades that infections (in particularly pneumonia and influenza)22,23,48 are associated with myocardial infarction. The high prevalence of infection (almost 20%) especially in patients with subacute ST suggests that infection plays an important role in the pathogenesis of subacute ST. Better surveillance and management strategies in order to timely identify the first symptoms of infection in hospitalised patients with a recently implanted coronary stent and strict compliance with current standards for the prevention followed by prompt antibiotic treatment of infections may aid in prevention.
Limitations
The results of our study should be interpreted in the light of the following limitations. First, the substantial period of time between the ST and the patient interview makes this population susceptible to information and recall bias. This may have resulted in both an underestimation as well as an overestimation of the prevalence of some triggering mechanisms (in particular vigorous exercise and emotional stress). However, this cannot explain the high prevalence of triggers found in this study. Moreover, exceptional activities or emotions in the hours preceding the catastrophic event of a ST are easy to remember. In addition, well-documented markers of infection and positive cultures as well as the reported time of performance of the emergent PCI for ST are not subjected to these forms of bias.
Second, the retrospective character of our study did not allow a case-crossover study design (cases serve as their own controls and therefore the design eliminates confounding by stable individual characteristics) because a detailed memory of the daily activities on just an ordinary day in the past (control-day) or a broader period of time is often not very clear. Due to the absence of this control group, a comparison with the prevalence of triggers in an average PCI-cohort cannot be made. Nonetheless, our data reveal a relatively high prevalence of triggering mechanisms in patients with ST as compared to the prevalence of similar triggering mechanisms in myocardial infarction (vigorous physical exercise: 3.8% to 10%16,28,33,46,49, any emotional stress: 4.4% - 6.8%26,28,49)
To overcome the aforementioned important limitations, very strict criteria were used to assign the presence of an exercise or emotional trigger. In addition, the interview was always started with an open question. Moreover, well-standardised reference charts and questionnaires from the field of sports medicine and psychology were used.
In conclusion, triggering mechanisms such as time of the day, physical exertion, emotional stress and infection may play an important role in a considerable number of patients presenting with ST. The prevalence of these triggering mechanisms is particularly high in patients with late and very late ST.
Acknowledgements
The present study was presented as an oral session during the American Heart Association Scientific Sessions in New Orleans (US) on November 11th 2008.
The sponsor, Sanofi Aventis, had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.

