In this chapter of Tools and Techniques Clinical, paravalvular leak closure is discussed using a stepwise approach. The following is a summarised overview of this technique. The complete, unabridged version with images is available online at http://www.pcronline.com/eurointervention/70th_issue/227.
Paravalvular leak (PVL) is a common problem for mechanical and bioprosthetic valves, occurring in 5-17% of implanted valves1. The associated aortic or mitral regurgitation can be associated with haemolysis or heart failure, leading to increased morbidity and mortality. Surgical treatment is associated with a high mortality risk, especially for redo surgery.
Indications for paravalvular leak closure include patients with significant regurgitation accompanied by symptoms of congestive heart failure and/or haemolysis. Important contraindications to PVL closure may include presence of active local or systemic infection, active ischaemia, mechanical instability of the prosthetic valve, intracardiac thrombus, and patients with a life expectancy due to comorbidities that is less than six months.
This paper will focus on aortic and mitral PVL, with a look at a few special situations regarding these valves.
Imaging and sizing
Imaging of paravalvular leak is difficult. Non-invasive methods (transthoracic echocardiography [TTE], transoesophageal echocardiography [TEE], three-dimensional echocardiography [3D echo], computed tomography [CT], magnetic resonance imaging [MRI]) all have distinct advantages and disadvantages. A variety of semiquantitative and quantitative parameters can be used to grade the jet for severity: these are discussed further in the European Society of Echocardiography Guidelines on Prosthetic Valve Disease2. Important features are location, size, calcification, and distance to other anatomic structures. It is our practice to use TTE and TEE for this information. With regard to location, aortic defects are described by location in relation to the coronary arteries or by a clock face; the mitral defects can be described by location or clock face. Angiography can be used for aortic leaks by varying the angle of the C-arm to find the optimal projection to visualise the leak. CT and MRI may provide new insights into PVL, but there is also the issue of calcification and other artefacts.
PVL sizing is often difficult, with leaks often taking a serpiginous course. Entry and exit orifices may be round, oval, or crescentic in shape. Size quantification can be performed by assessing the vena contracta size or by using 3D echo to assess the length and width of the orifice. Figure 1-Figure 3 show examples of imaging and sizing for PVL.
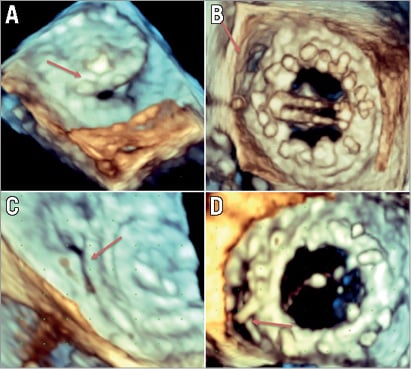
Figure 1. Shape of paravalvular leaks. Paravalvular leaks can take a variety of shapes. These include round (A), oval (B), slit-like (C) and crescentic (D).
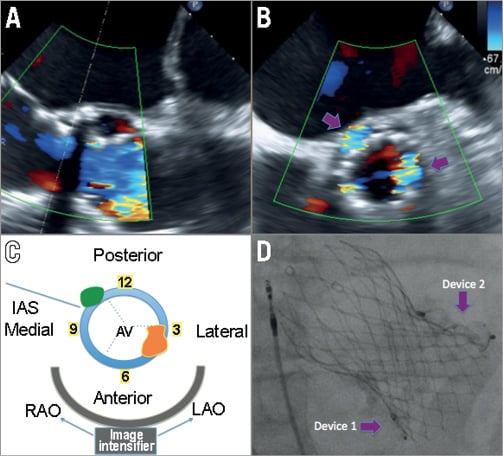
Figure 2. Location and sizing of aortic paravalvular leaks. Aortic paravalvular leak in a patient after CoreValve (Medtronic , Minneapolis, MN, USA) TAVI implantation. Here we see one aortic valve in 120-degree view (A), which, on biplane view (B), has at least two areas of major leak (purple arrows). A schematic of part B is shown in C, where the leaks are shown in relation to the interatrial septum (IAS) and the aortic valve (AV). One leak, which is green, is located near the IAS at the 10-11 o’clock position on the clock face, which is also posterior and medial. This would be in the region of the non-coronary cusp of the original AV. The other leak, which is orange, is located near 4 o’clock on the clock face, is mostly lateral, and located in the region of the original right coronary cusp. The bottom of panel C shows how the image intensifier would visualise the lesion. An extreme LAO angulation would overlap the orange and green PVL above each other. However, an RAO angulation would allow both to be seen adjacent to the valve. In D, we see a post-procedure RAO angulation on fluoroscopy, with two devices in each of the major paravalvular leaks on each side of the CoreValve.
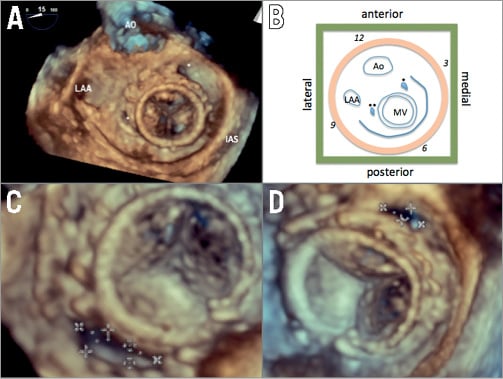
Figure 3. Location and sizing of mitral paravalvular leaks. This patient had two mitral paravalvular leaks (asterisk) at various locations in relation to the aorta (Ao), left atrial appendage (LAA) and interatrial septum (IAS) (A). In the schematic shown in B, one can see that the anatomy is distorted, with the mitral valve being more medial compared to normal anatomy. The two leaks are seen as well. On the clock face (orange disc) with the aortic valve at the 12 o’clock position, one leak is at the 2 o’clock position (one asterisk) and another leak is at the 9 o’clock position (two asterisks). In relation to spatial position, one leak is anterior and medial (one asterisk) and the other leak is lateral (two asterisks). This illustrates the difficulty of using one naming system, especially in distorted valve anatomy after replacement. The lateral leak is shown in C as a large crescentic leak, with measurements of 16×6 mm. The anteromedial leak is shown in D, with a measurement of 9×3 mm.
Access
There are three main methods for access, namely transfemoral, transseptal, and transapical. Transfemoral retrograde access is often used for aortic or medial mitral paravalvular leaks (radial access can be considered for small leaks that require small sheaths, but transfemoral access allows more options). Transseptal access (obtained under both fluoroscopic and transoesophageal echocardiographic guidance to find an inferior and posterior septal position) can be useful for mitral paravalvular and difficult-to-cross aortic paravalvular leaks. Transapical access is used for difficult-to-reach mitral leaks or in patients with both mechanical aortic and mitral valves; in this case an occluder device can be used to close the access site. In each of these situations, the leak is crossed with a wire, a catheter crosses the leak over the wire, and the wire is switched for a stiffer wire. Appropriate heparin should be given and ACT should be monitored to be between 250 and 300 s. Paravalvular leak closure can be performed with conscious or general sedation. At our centre, we have significant experience with long-term TEE use without or with only very mild sedation in awake patients. This is left to the discretion of the centre.
Device selection
A device is selected based on leak size and shape. Where possible, a device should be chosen that closes the defect entirely. Commonly used devices include the Amplatzer™ (St. Jude Medical Inc., Minneapolis, MN, USA) closure devices for paravalvular leak closure. It is rare that the device closes the defect entirely, because it does not match a unique defect shape or size. Multiple devices can be used, but this increases the risk of infection and impinging on leaflet motion. This points to the need for more geometrically appropriate devices to treat this condition.
In the light of this, we have made a general paradigm as to what device to select for what type of leak. For a small cylindrical leak, we will often use an Amplatzer Vascular Plug II (AVP II) or patent ductus arteriosus (PDA) occluder. For an oval leak, an AVP III occluder is preferred. For a small leak with significant angulation and small neck, an AVP IV occluder can be considered. Sizing of these devices comes most often from echocardiographic measurements, both in 2D and 3D. Angiography can also be useful for aortic PVL, which can be measured in profile with appropriate C-arm angulation. The size dictates the device, which dictates the sheath/guide used for delivery. We often use Cook Shuttle sheaths (Cook, Bloomington, IN, USA) or sheaths that will help deliver the guide catheters to the appropriate location. We do not recommend using external catheter size to measure leak size, as a variety of factors, e.g., calcification and tortuosity, can cause difficulty in the catheter’s ability to cross the leak. However, once a catheter has successfully crossed the leak, echocardiography can be used to evaluate the regurgitant jet with the catheter in place, which can occasionally give an idea of volume within the leak. Balloon sizing is not recommended, as this carries the risk of balloon rupture within the leak.
Aortic paravalvular leak
For aortic paravalvular leak, the preferred route is retrograde transfemoral access. Catheters that are often helpful include Judkins Right 4 (JR4), Multipurpose (MP), or Amplatz-1 catheters with the use of a hydrophilic 0.035” wire. Once the defect has been crossed with the catheter, the wire can be exchanged for a stiff support 0.035” wire. This is then used to exchange the catheter for a long sheath or guide catheter (the size of which is determined by the device). Once the sheath or catheter is in place, the device is delivered. Echocardiography is used to monitor for interval change in the regurgitant jet. Other important issues to rule out are leaflet obstruction and coronary ostial obstruction. This can be performed with fluoroscopy and root angiography, respectively. Echocardiography can be used to make sure there is no interference with the mitral valve apparatus.
If there are issues due to leak tortuosity or irregular borders of the leak, a rail can be used for support. This can be done either with transseptal access and snaring the wire in the left atrium or by transapical access and externalising the wire out of the left ventricular apex. Figure 4 shows examples of aortic PVL closure.
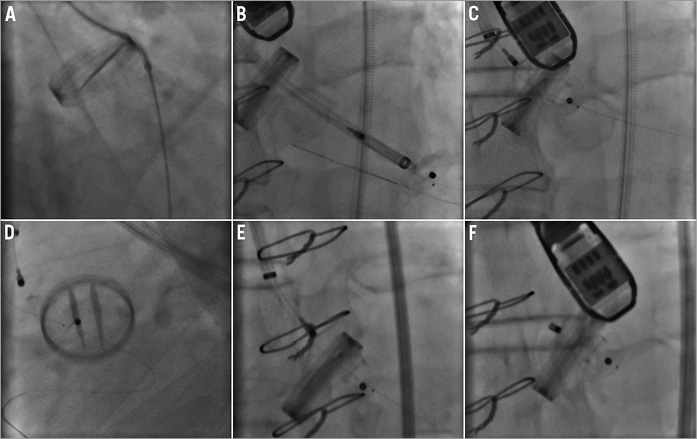
Figure 4. Aortic paravalvular leak closure: single device. A 74-year-old male with paravalvular leak in relation to mechanical aortic valve. The leak was an eccentric leak in the region of the non-coronary cusp and measured 10 x 3 mm on the echocardiogram. Right femoral arterial access was obtained and a 5 Fr sheath was placed. A 5 Fr AL1 guide catheter and Terumo hydrophilic 0.035” wire were used to cross the leak in a retrograde fashion (A). This catheter was exchanged over an Amplatz Extra Stiff wire for a 7 Fr Cook Shuttle sheath, which was placed in the left ventricle (B). A 0.014” Iron Man wire (Abbott Vascular, Santa Clara, CA, USA) was placed as access protection through the paravalvular leak into the left ventricle. A 10 mm PDA occluder device was tried but was unsuccessful in closing the defect (C) and was removed. The 0.014” wire stayed in place (D). A 4 Fr 125 cm JR4 catheter was placed in a coaxial fashion inside the Shuttle sheath to traverse the paravalvular leak over the 0.014” wire and re-establish the Shuttle sheath across the leak (not pictured). Then a 12/3 mm AVP III device was placed across the leak (E). After fluoroscopic and echocardiographic confirmation of minimal leak and good valve function, the device was released (F).
Once the device is in place, it is important to rule out complications from the device. The prosthetic aortic valve should be evaluated, preferably both by echocardiography and angiography, to make sure leaflet movement is not compromised (Figure 4). If the device is in the area of the former left and right coronary cusp, coronary patency should be shown, either by selective or root angiography or by TEE. If the device is in the area of the former non-coronary cusp, the anterior mitral valve leaflet should also be evaluated.
Device success consists of a decrease in aortic regurgitation and improvement in symptoms. Regular follow-up with TTE can be performed pre-discharge, at six months, 12 months, and annually thereafter with earlier follow-up if symptoms occur. Regular haemoglobin and haematocrit levels may be checked to rule out haemolysis at these intervals.
Mitral paravalvular leak
Mitral leak should be well evaluated by TEE to determine position, size, and shape. We approach mitral leaks first using a retrograde transfemoral approach with a 5 Fr JR4 or internal mammary (IM) catheter and a 0.035” hydrophilic guidewire. This is then used to cross the leak and then the wire is exchanged for a stiff 0.035” wire. This is then used to advance a delivery sheath or guide catheter (the size of which is determined by the device to be used). The device is then delivered.
TEE is used to monitor for interval change in the regurgitant jet. Another important issue is to make sure the mitral leaflets are mobile and free from obstruction. The pulmonary veins should also be free from obstruction. If the device is placed anteriorly, the aortic valve and left ventricular outflow tract should also be evaluated to rule out interference from the device. 3D TEE can be used to make sure the device is oriented correctly in relation to the valve.
If the retrograde transfemoral approach is unsuccessful at crossing the leak, an antegrade transseptal approach is used. The preferred crossing point is superior and posterior for both lateral and medial mitral leaks. Once the 0.035” hydrophilic wire crosses the leak, a 5 Fr JR4 or MP catheter can be advanced over the wire to cross the leak. (Of note, if there is difficulty in finding a catheter that will allow the wire to cross the defect, an Agilis™ steerable introducer [St. Jude Medical Inc., Minneapolis, MN, USA] may be used). This is then exchanged over an Amplatz Extra Stiff wire (with a hand-made curve) in the left ventricle for a larger guide catheter or sheath (e.g., Cook Shuttle sheath). Once this is in place, the device can be advanced through the guide catheter/sheath to the appropriate position and deployed.
If the catheter does not cross via the antegrade transseptal approach, the next step is a rail. This is done by passing a long 300 cm wire through a catheter in a retrograde fashion through the defect, snaring the wire in the left atrium using a snare and a second catheter, externalising the wire in the femoral vein, exchanging the wire for a long Amplatz Extra Stiff wire, and then advancing the sheath/guide catheter through the defect over the same wire. Often the transseptal sheath is advanced through the defect itself.
Once the device is in place, it should be evaluated closely by TEE. It is important to make sure that the valve leaflets are mobile (Figure 5). If the device is placed anteriorly, the aortic valve should also be evaluated. 3D TEE can be used to make sure the device is oriented correctly in relation to the valve. TEE is necessary to show change in the regurgitation volume with the device in place. It is important to try to achieve as complete a closure as possible. After this issue has been satisfactorily addressed, the device may be released.
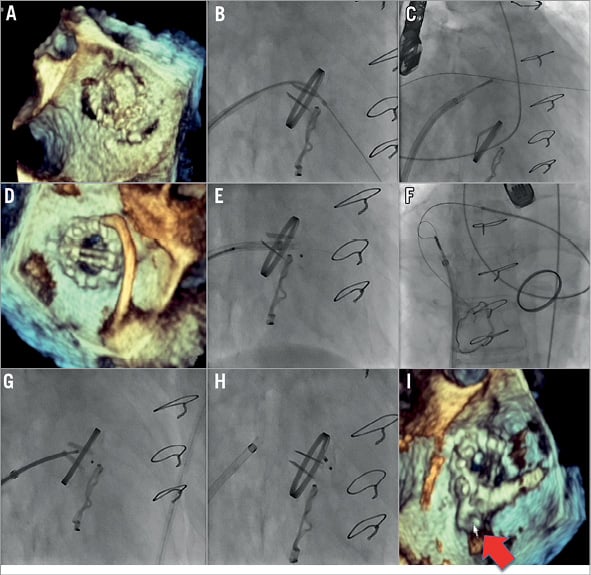
Figure 5. Mitral paravalvular leak closure: one device. Several years after placement of a mechanical mitral valve prosthesis and tricuspid ring, the patient developed severe mitral regurgitation with reversal of flow in pulmonary veins. This was due to two paravalvular mitral leaks, one anterolateral (orifice 6×15 mm) and one posterior (orifice 10×8 mm). The anterolateral leak was close to a section of tissue that interfered with leaflet motion; therefore, the decision was made to pursue only the posterior leak. Rather than approach the leak in a retrograde fashion, the decision was made to approach the leak in an antegrade fashion due to presumed easier access to the posterior leak. After placement of a 5 Fr sheath in the right femoral artery and a 9 Fr sheath in the right femoral vein, transseptal puncture was performed. Antegrade crossing was obtained (wire and diagnostic catheter) but the sheath did not cross (B). Therefore, a 5 Fr JR4 catheter and 0.035” Terumo wire were introduced through the aorta and the leak was crossed in a retrograde fashion; the Terumo wire was advanced and snared through a transvenous/transseptal sling in the left atrium and externalised (C, D). This allowed the transseptal 9 Fr sheath to be advanced through the leak. A 10 mm Amplatzer muscular VSD occluder was brought through the leak but caused mitral valve impingement (E). Therefore, this was removed and retrograde access from the aorta and sling in the left atrium was again obtained (F). Next an Amplatzer 10 mm PDA occluder was placed across the defect (G, H), which did not interfere with mitral leaflet movement. Echocardiography showing final device position (I).
Device success consists of a decrease in mitral regurgitation and improvement in symptoms. The patient should be followed closely with pre-discharge TTE, six-month TEE, 12-month TTE, and annual transthoracic echocardiography. If there are symptoms, earlier follow-up is warranted. Regular haemoglobin and haematocrit levels may be checked to rule out haemolysis at these intervals.
Long-term results
Technical success is correct deployment of the device without significant residual regurgitation, new prosthetic valve malfunction, haemolysis, or interference with other cardiac structures. Clinical success is improvement in NYHA functional class by at least one grade and/or improvement in mechanical haemolysis. It is important to watch for complications from the procedure, which can occur in 0.7-4% of cases3. These include valve interference, device embolisation, stroke, endocarditis, post-procedural haemolysis, and device erosion.
Conclusion
The treatment of paravalvular regurgitation requires careful preprocedural imaging, planning and patient selection. Through principles of access, technique, and device choice, it is possible to achieve both technical and clinical success.
The online version of this paper provides a more detailed, in-depth description of each aspect in the pursuit of a successful procedure.
Acknowledgement
Echocardiographic images courtesy of Ilona Hofmann and Laura Vaskelyte, CardioVascular Center, Frankfurt, Germany.
Conflict of interest statement
H. Sievert has received study honoraria, travel expenses, and/or consulting fees (modest) from Abbott, Access Closure, AGA, Angiomed, Aptus, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, Covidien, CSI, CVRx, EndoCross, ev3, FlowCardia, Gardia, Gore, Guided Delivery Systems, InSeal Medical, Lumen Biomedical, HLT, Lifetech, Lutonix, Maya Medical, Medtronic, NDC, Occlutech, Osprey, Ostial, PendraCare, pfm Medical, Recor, ResMed, Rox Medical, SentreHeart, Spectranetics, SquareOne, Svelte Medical Systems, Trireme, Trivascular, Venus Medical, Veryan, and Vessix. He has received grant and research support from Cook and St. Jude Medical. He owns stock options in Cardiokinetix, Access Closure, Velocimed, Lumen Biomedical, Coherex, and SMT. The other authors have no conflicts of interest to declare.

