
Abstract
Aims: Meticulous lesion preparation prior to bioresorbable vascular scaffold (BVS) implantation has been strongly recommended. The aim of this study was to investigate if there was a benefit associated with scoring balloon use in lesion preparation in comparison to conventional balloons prior to implantation of a BVS.
Methods and results: Of the lesions treated with BVS between May 2012 and July 2014, 155 lesions in the conventional balloon group and 29 lesions in the scoring balloon group were included. Procedures without predilatation and those which utilised cutting balloon or rotational atherectomy devices were excluded. Complex (B2/C lesion: 76.1% vs. 93.1%; p=0.028), restenotic (5.2% vs. 17.2%; p=0.036) and calcified (36.1% vs. 79.3%; p<0.001) lesions were more common in the scoring balloon group. Compared to the conventional balloon group, the scoring balloon group demonstrated better procedural IVUS outcomes with regard to both expansion index (defined as scaffold lumen area divided by final post-dilatation balloon cross-sectional area, 0.71 vs. 0.86; p<0.001) and eccentricity index (defined as minimal scaffold diameter divided by maximal scaffold diameter, 0.78 vs. 0.84; p<0.001). The occurrence of ischaemia-driven target lesion revascularisation at one year was similar (6.1% vs. 7.1%; p=0.87).
Conclusions: Lesion preparation for complex lesions using a scoring balloon appeared to facilitate optimal sizing and radially concentric expansion of BVS.
Abbreviations
BVS: bioresorbable vascular scaffold
IVUS: intravascular ultrasound
MLA: minimal lumen area
MLD: minimal lumen diameter
OCT: optical coherence tomography
QCA: quantitative coronary angiography
TLR: target lesion revascularisation
Introduction
The second-generation Absorb bioresorbable vascular scaffold (BVS 1.1; Abbott Vascular, Santa Clara, CA, USA) has an entirely novel composition, characterised by its eventual resorption after implantation1-3. These beneficial characteristics may address the limitations of late stent thrombosis associated with current-generation drug-eluting metallic stents whilst permitting future coronary bypass surgery if required. Studies to date have been promising with regard to clinical efficacy and safety with no serious concerns4-6. However, due to their restricted use in the setting of initial clinical trials5,7, their applicability to complex lesions including diffuse lesions, small vessel disease, bifurcations and calcified lesions remains to be established. By virtue of thicker struts (157 μm) and crossing profile (1.4 mm) in comparison to metallic stents, BVS are more difficult to deliver. This is particularly relevant when treating complex lesions8. Previous studies have demonstrated excellent conformability9,10, numerically higher acute recoil (albeit insignificant)11 and reduced cross-sectional concentric expansion of scaffolds12. Consequently, optimal lesion preparation prior to BVS implantation has been encouraged to facilitate their successful delivery and adequate expansion. In this context, particularly where lesions are resistant to conventional balloon dilatation, plaque-modifying devices including rotational atherectomy, cutting balloons and scoring balloons prior to BVS implantation may be critical for obtaining an acceptable procedural result13.
We hypothesised that patients treated with BVS following lesion preparation using scoring balloons would have better scaffold expansion, lumen size and radial concentricity in comparison to patients where conventional balloons alone were utilised. The aim of this study was therefore to investigate if there was a benefit associated with scoring balloon use in lesion preparation in comparison to conventional balloons prior to BVS implantation, aided by intravascular ultrasound (IVUS) assessment.
Methods
STUDY POPULATION AND METHODS
Patients treated with BVS between May 2012 and July 2014 in two Italian centres (EMO-GVM Centro Cuore Columbus and San Raffaele Scientific Institute, Milan, Italy) were retrospectively identified. Written informed consent was obtained from each patient for both the procedure and subsequent data collection. All patients who underwent predilatation prior to BVS implantation utilising the AngioSculpt® scoring balloon (AngioScore, Fremont, CA, USA) (with or without prior conventional balloon use) were assigned to the scoring balloon group, whereas all patients treated with a conventional balloon alone were allocated to the conventional balloon group. Procedures without IVUS guidance, without predilatation and those that utilised cutting balloon or rotational atherectomy devices were excluded. The scoring balloon was also specifically used in a subset of patients with heavily calcified lesions (assessed by angiography or IVUS) or following inadequately dilated lesions using conventional balloons. Concomitant use of IVUS in each procedure was at the operator’s discretion. For intravascular imaging during the procedure, the Atlantis™ SR Pro2 40 MHz coronary imaging catheter with automatic motorised pullback of 0.5 mm/s in combination with iLab Ultrasound Imaging System (both Boston Scientific, Marlborough, MA, USA) was employed. Uninterrupted continuation of dual antiplatelet therapy for 12 months following BVS implantation was recommended for all patients.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
QCA utilising the QCA-CMS 5.2 system (Medis medical imaging systems bv, Leiden, The Netherlands) was performed by an experienced interventional cardiologist blinded to operator strategy and procedural outcomes. Angiographic variables including lumen diameter and reference vessel diameter were selected on the basis of the minimal lumen diameter (MLD) at index angiography. The same site of interest was then used for final angiographic evaluation.
QUANTITATIVE IVUS ANALYSIS
Two-dimensional greyscale IVUS analysis, with the use of collected digital raw data, was performed offline by a skilled cardiologist completely independent of values obtained on-site. Variables before and after intervention with regard to lumen, vessel and scaffold properties were derived from a single anatomical location as defined by the minimal lumen area (MLA) on the basis of pre-IVUS imaging. Minimal scaffold area (MSA) was not used as the post-procedural measurement site in the study analysis due to this site not necessarily being the same site as the pre-intervention MLA location and not necessarily having been predilated by the scoring balloon in the setting of a long segment of disease. When pre-interventional imaging could not be performed, for example in the setting of total coronary occlusion or when the IVUS catheter could not be advanced, only post-procedural variables were acquired.
CLINICAL OUTCOMES
Ischaemia-driven target lesion revascularisation (TLR) at 360 days was evaluated with the Kaplan-Meier method, and between-group differences were compared.
DEFINITIONS
In order to minimise the effects of differences in the size of the vessel, balloon and stent in each individual case, dimensionless indices were introduced as follows: 1) plaque eccentricity index; 2) expansion index, which represents the ratio of the actual final scaffold area to the expected one as estimated by the nominal diameter of the final balloon – therefore a higher value indicates better expansion; and 3) eccentricity index, where a higher value denotes more concentric expansion of a BVS in cross-section (Figure 1)14-17.
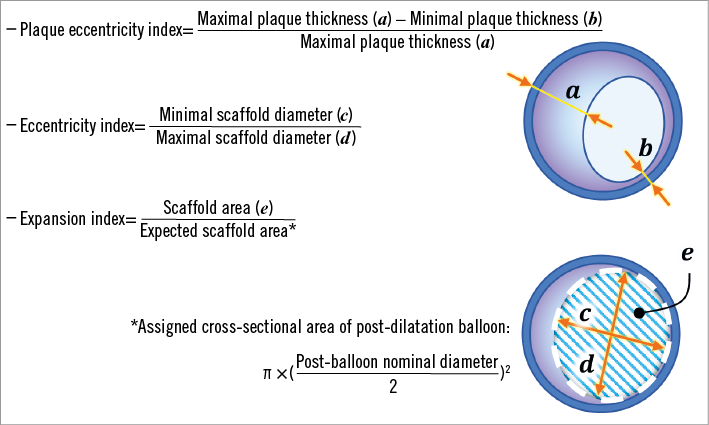
Figure 1. Formulae and schematics for indices of intravascular ultrasound.
Staged procedures performed within eight weeks were deemed to be a single procedure. By using the IVUS substudy of the ABSORB cohort A and B trial as a reference12, a lesion was classified as a calcific lesion when an arc of calcium was ≥90° on IVUS at the site of the MLA. We also evaluated the severity of the coronary artery disease using the angiographic scoring system according to the Synergy between PCI with TAXUS and Cardiac Surgery (SYNTAX) trial18. Stent thrombosis was defined as definite, probable, or possible according to the Academic Research Consortium19.
STATISTICAL ANALYSIS
Descriptive statistics of patient, lesion and procedural characteristics are presented. Categorical variables are reported as counts and percentages, and compared between groups by chi-square test or Fisher’s exact test. Continuous variables are presented as means with standard deviation and were compared with the two-sample Student’s t-test. The Kaplan-Meier method was used to estimate the one-year occurrence of ischaemia-driven TLR in each group, and the log-rank test to compare between-group differences. SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
BASELINE CHARACTERISTICS
A total of 266 lesions (177 patients) treated with BVS were initially screened. Twenty-three lesions were excluded due to lack of lesion preparation or preparation with methods other than with the use of conventional balloon alone or AngioSculpt predilatation. An additional 59 lesions were excluded due to no (or suboptimal) IVUS imaging lesion data. Therefore, 184 lesions (130 patients) were included in the final analysis, 155 lesions (106 patients) in the conventional balloon group and 29 lesions (24 patients) in the scoring balloon group (Figure 2).
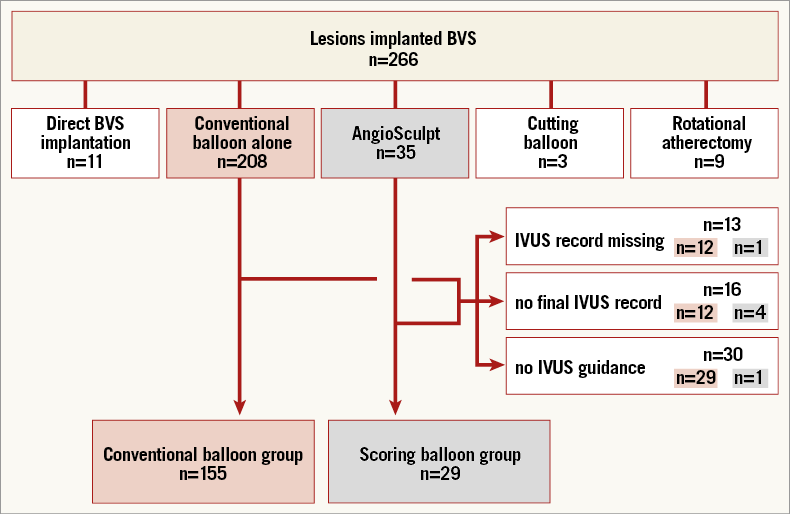
Figure 2. Flow diagram of patient allocation.
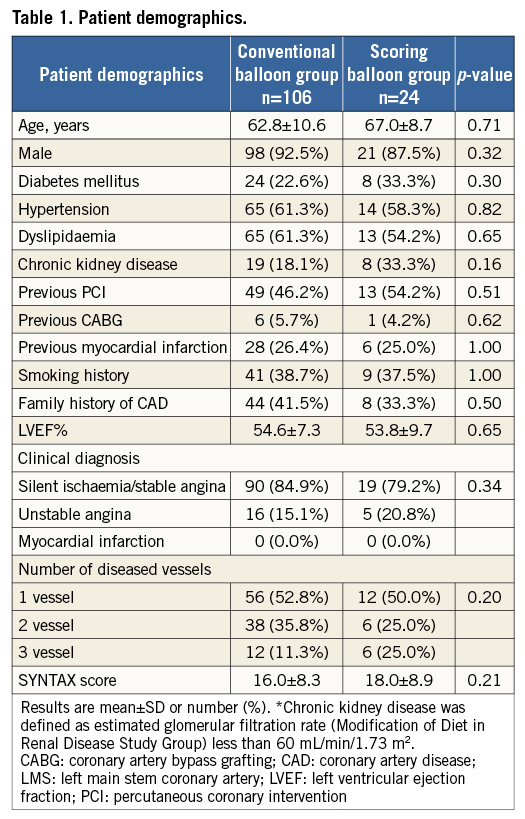
The median follow-up period of patients was 395 days (interquartile range 208.8-513.3). The mean age was 62.8 (±10.6) years and 67.0 (±8.7) years in the conventional and scoring balloon groups, respectively (p=0.71). Other patient characteristics including coronary risk factors were similar between the two groups (Table 1).
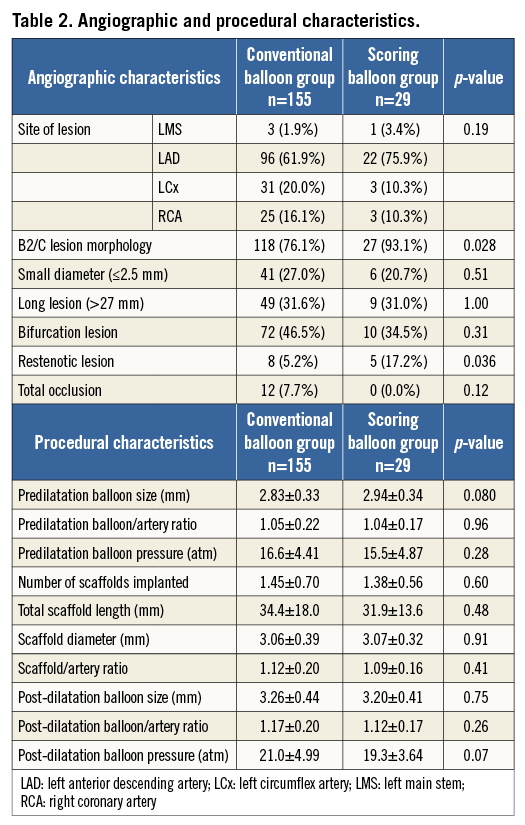
Lesion complexity (B2 or C lesion) was more severe in the scoring balloon group (conventional balloon vs. scoring balloon, 76.1% vs. 93.1%; p=0.028). BVS implantation in the setting of restenotic lesions (5.2% vs. 17.2%; p=0.036) and calcific lesions (36.1% vs. 79.3%; p<0.001) was more frequent in the scoring balloon group (Table 2). The number of implanted stents and total stent length were similar between the two groups. Although larger diameter balloons were utilised for predilatation in the scoring balloon group (2.83±0.33 mm vs. 2.94±0.34 mm; p=0.08), the balloon-artery ratio was comparable between groups. All BVS implantations were followed by additional post-dilatation using a non-compliant balloon, with a tendency towards higher pressure post-dilatation in the conventional balloon group (21.0±5.0 atm vs. 19.3±3.6 atm; p=0.07). All pre-procedural QCA variables were similar between groups (Table 3). Pre-interventional IVUS prior to initial predilatation was performed in 126 lesions (100 lesions [64.5%] vs. 26 lesions [89.7%]); however, all lesions were evaluated by IVUS prior to definitive implantation of the BVS. As assessed by IVUS, the angle of the arc of calcium at the site of MLA was 62.8° on average in the conventional balloon group but 167.3° in the scoring balloon group (p<0.001) (Table 3). Plaque eccentricity was comparable between groups.
POST-INTERVENTIONAL QCA AND IVUS ANALYSIS
There were no differences with regard to QCA variables between the two groups. However, IVUS variables of both expansion index (0.71±0.18 vs. 0.86±0.20; p<0.001) and eccentricity index (0.78±0.11 vs. 0.84±0.07; p<0.001) were higher in the scoring balloon group (Table 3, Figure 3).
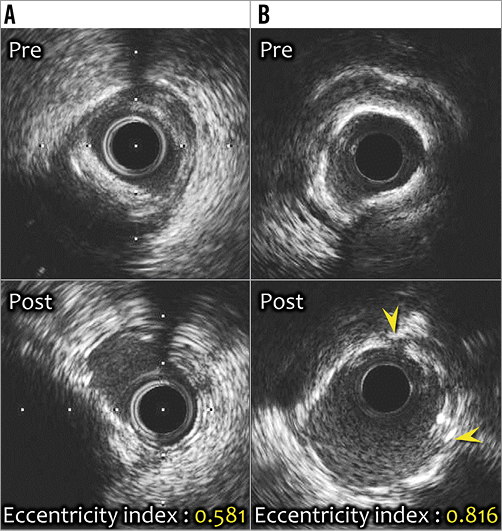
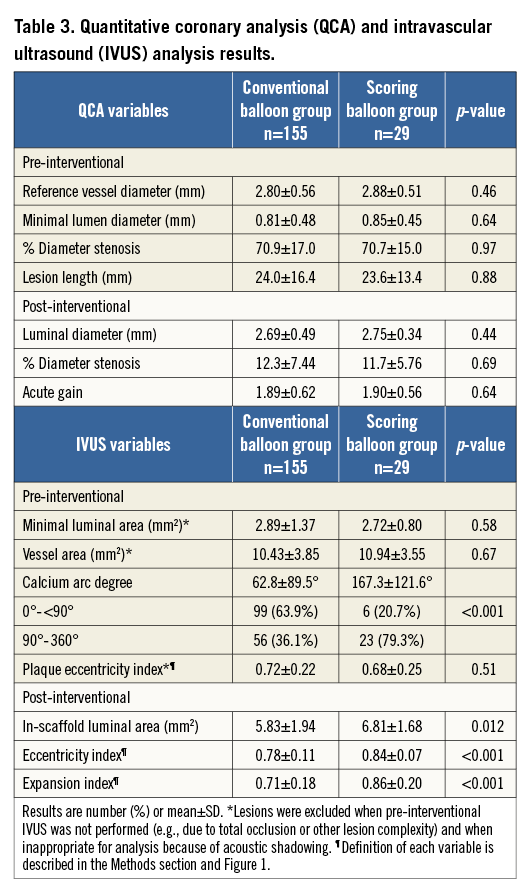
Figure 3. Representative intracoronary images. A) Eccentric calcified lesion (upper panel). The bioresorbable vascular scaffold (BVS) was implanted following predilatation with a conventional balloon alone (lower panel). Despite additional high-pressure (22 atm) post-dilatation with a non-compliant balloon, there was eccentric expansion of the BVS with greatest expansion at sites with minimal calcium (eccentricity index: 0.581). B) Significant stenotic lesion accompanied by 316° calcification (upper panel). Optimal BVS expansion following scoring balloon dilatation was achieved (lower panel). Small crack (yellow arrowheads) noted on the calcium deposit may have contributed to the ability of the scaffold to expand concentrically (eccentricity index: 0.816).
Analysis of the calcified lesion subset demonstrated that lesions predilated with conventional balloons were associated with less concentric expansion of scaffolds when compared to non-calcified lesions (p=0.007). On the other hand, predilatation with a scoring balloon resulted in comparable concentric expansion in both calcified and non-calcified lesions (p=0.58) (Figure 4). QCA assessment of lesions which were treated with a scoring balloon of the same diameter as the preceding conventional balloon demonstrated that the additional scoring balloon dilatation resulted in a larger lumen diameter than that of the preceding conventional balloons (1.49±0.49 mm vs. 1.92±0.54 mm; p<0.001) (Figure 5).
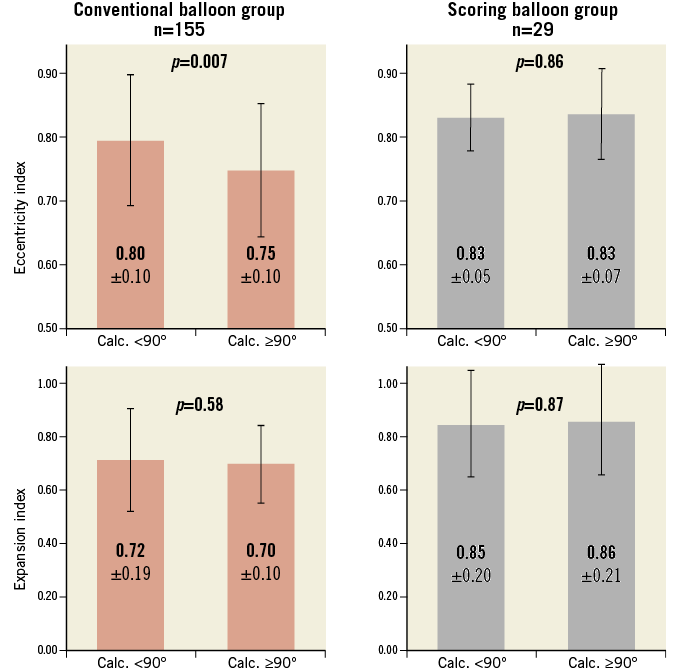
Figure 4. Intragroup comparison of eccentricity index and expansion index. Eccentricity index and expansion index were compared between non-calcified and calcified subsets (defined as an arc of calcium <90° or ≥90° on IVUS at the site of the MLA) within each group. Within the conventional balloon group, 99 vs. 56 patients were compared in the non-calcified vs. calcified subset, whereas 6 vs. 23 patients were compared within the scoring balloon group. Values represented are mean±SD, and between-subset differences were calculated by dependent t-test.
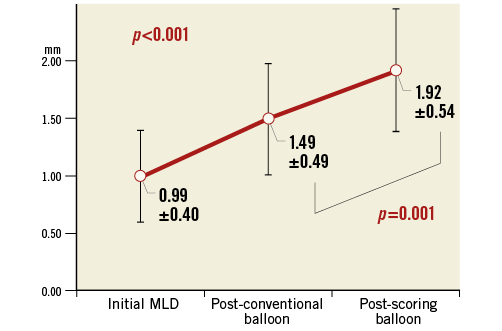
Figure 5. Increases in lumen diameter on QCA at each step of the predilatation. Comparison of lumen diameter of lesions predilated with the use of a conventional balloon and then subsequently with an AngioSculpt scoring balloon of the same diameter (n=24). Values are mean±SD. Overall differences were calculated with repeated measures analysis of variance. The result of the post hoc Tukey’s honest significant difference test is also expressed. MLD: minimal lumen diameter; QCA: quantitative coronary angiography
CLINICAL OUTCOMES
The estimated occurrence of ischaemia-driven TLR at 360 days in each group was similar (6.1% vs. 7.1%; p=0.87) (Figure 6). There were no cases of stent thrombosis.
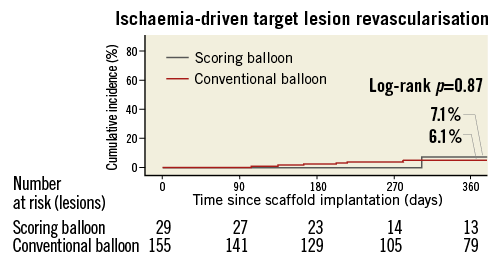
Figure 6. Kaplan-Meier cumulative event curves for ischaemia-driven target lesion revascularisation at 360 days. Estimated event rates were calculated by the Kaplan-Meier method, and differences between groups were calculated with the log-rank test.
Discussion
The principal findings of the current study were as follows: 1) AngioSculpt scoring balloon dilatation prior to BVS implantation resulted in greater concentric expansion of scaffolds that reflected better the true lumen size in comparison to when conventional balloons alone were used; 2) additional AngioSculpt scoring balloon dilatation following a preceding conventional balloon of the same diameter resulted in an increased lumen area before BVS implantation; and 3) the incidence of TLR at one year was similar between the two groups.
These results were obtained despite more adverse lesion characteristics for patients treated with the AngioSculpt scoring balloon.
Ever since the era of bare metal stents, many studies have supported the concept that “bigger is better” in terms of metallic stent implantation20-22. These conclusions may also be true for polymeric bioresorbable scaffolds. In order to obtain the greatest achievable final lumen area, a strategy of delivering maximal concentric radial expansion would appear to be the most reasonable approach. In order to prevent damage or rupture to both the scaffold and the coronary artery, it is crucial to avoid excessive ellipsoid expansion of scaffolds that exceeds the maximal dilatable diameter of the BVS or underlying vessel23. Additionally, effective plaque modification facilitates delivery of BVS and better expansion. Current-generation BVS have a relatively bulky profile with thick struts (157×191 µm) and lower radial force, and, as a consequence, have numerically higher acute recoil (6.7±6.4% vs. 4.3±7.1%)11, and more eccentric radial expansion (0.85±0.08 vs. 0.90±0.06; p<0.001)12 when compared to the XIENCE V® metallic stent (Abbott Vascular). Taking these features and late lumen loss into account, aggressive lesion preparation with high-pressure post-dilatation, especially in the setting of complex lesions, is strongly encouraged to achieve optimal procedural results24,25.
The AngioSculpt scoring balloon has three nitinol spiral elements surrounding the surface of a semi-compliant balloon. This enables the focused homogenous transmission of pressure through these elements resulting in “scoring” of the plaque irrespective of the severity and distribution of calcification and theoretically with a reduction in the risk of catastrophic dissection. This subsequently facilitates symmetric and effective expansion of BVS struts as illustrated in Figure 4. As demonstrated in Figure 5, predilatation using a scoring balloon probably results in reduced elastic recoil, when compared to the use of a conventional balloon alone. This may help deployment of a BVS at the site of the target lesion. The larger expansion index noted in the scoring balloon group in the current study also suggests that the use of a scoring balloon may assist in BVS post-dilatation. In addition, on the basis of IVUS imaging, we did not identify any incidences of scaffold fracture, which may also have had an impact upon acute recoil.
Currently available rotational atherectomy devices might provide the most effective plaque modification; however, this does require specialist equipment and operator procedural expertise, and is associated with an increased risk of complication26-28. Cutting balloons are also an alternative option but again they are associated with procedural risks, including coronary perforation or aneurysm, and have a larger profile29,30. AngioSculpt, however, is currently limited by a higher monetary cost in comparison to conventional balloons.
The mean eccentricity index of 0.80 of BVS in our patient cohort was lower when compared to previously reported measurements of metallic stents (0.86-0.92)31,32, supporting the study by Brugaletta et al12. However, reflecting the complexity of this “real-world” study population, only 6.0% would have met the inclusion criteria of the ABSORB cohort B trial, and therefore one might have expected an even worse eccentricity index7. In spite of this, in our study, patients within the scoring balloon group demonstrated an eccentricity index of 0.84±0.07 which was comparable to the patients treated with BVS in the study by Brugaletta et al.
It is currently unclear whether eccentric radial expansion may lead to undesirable clinical outcomes. Kaneda et al failed to demonstrate a reduction in intimal hyperplasia following implantation and concentric expansion of sirolimus-eluting stents at follow-up as assessed by IVUS31. In another study using OCT, Otake et al also did not demonstrate a relationship between cross-sectional eccentricity of stent expansion and neointimal thickness. However, greater eccentricity was associated with a greater occurrence of uncovered struts, which was related to OCT appearances consistent with thrombus formation32. These findings supported previous observations with the use of bare metal stents where a significant difference in radial stent symmetry on IVUS was noted between the subacute stent thrombosis group and a matched control group33. In addition, another drug-eluting stent study by Liu et al demonstrated that greater stent asymmetry as defined by IVUS was associated with greater in-stent thrombosis when compared to patients who suffered no events or patients in the in-stent restenosis group34.
In summary, although eccentric radial expansion of struts may not have an impact upon subsequent neointimal hyperplasia, late lumen loss and resultant TLR, it may increase the risk of thrombus-related events as a consequence of uncovered struts32,33.
Despite better procedural outcomes with regard to both greater expansion and eccentricity index in the scoring balloon group, the current study did not demonstrate that this translated into improved clinical outcomes with respect to ischaemia-driven TLR. No patients involved in the present study experienced stent thrombosis and we were therefore unable to explore the relationship between eccentric expansion and the occurrence of thrombotic events.
In summary, based on observations from previous studies, the larger stent/scaffold area may lead to a reduction in the occurrence of TLR, whilst concentric, circular radial expansion may reduce the risk of future thrombotic complications.
Study limitations
Patients recruited to this study were not randomised. All decisions including the indication for BVS, the use of IVUS guidance, the concomitant use of any other devices and the utilisation of information obtained were left to operator discretion. Given the small sample size, the study was not adequately powered to detect differences in clinical outcomes. There may be a risk of underestimation of the expansion index due to substitution of the expected stent diameter by the nominal diameter of the post-dilatation balloon that did not take the final dilatation pressure into account. Finally, follow-up angiography was not routinely mandated and thus intracoronary imaging at this latter time point was not acquired. Future studies with a larger sample size, possibly also including non-complex lesions with longer follow-up including angiography and intravascular imaging, are eagerly awaited to investigate whether the use of scoring balloon predilatation results in optimal outcomes.
Conclusions
The current study suggests that lesion preparation using the AngioSculpt scoring balloon may reduce acute recoil prior to BVS deployment, facilitate optimal sizing and result in improved radial concentric expansion of BVS, even in the setting of complex and calcified lesions. Larger clinical studies are needed to demonstrate that these improvements in scaffold expansion will lead to clinical benefits.
| Impact on daily practice The use of BVS is increasingly being expanded from simple lesions to those with more complex characteristics. Our study suggests that the use of a scoring balloon for lesion preparation in comparison to conventional balloons prior to BVS implantation may lead to more concentric BVS expansion following implantation, as assessed by intravascular imaging. These data support the use of a scoring balloon for lesion preparation when contemplating BVS treatment of complex lesions. |
Conflict of interest statement
A. Latib is a consultant for Medtronic. The other authors have no conflicts of interest to declare.

