Introduction
Despite the advancement in percutaneous interventional procedures including newer stents and better delivery systems, the failure to deliver a stent to the target lesion, especially in arteries with complex anatomy, remains a common problem. Various techniques have been used to solve or rather help with this dilemma including straightening the artery with a second “buddy” wire1 or “buddy” balloon, larger and more supportive guiding catheters, or deep intubation with the guiding catheter for back up support. The Heartrail II (Terumo, Tokyo, Japan) “five in six catheter system” also called ”mother and child”, involving the insertion of a flexible tipped extra length (120 cm) 5 Fr catheter for deeper intubation with extra back-up support, has been described in the literature, and is an accepted technique for improving support and delivering stents in difficult cases2-5. More recently, a new “child” support catheter has been introduced: the GuideLiner™ (Vascular Solutions, Minneapolis, MN, USA). The device received CE marking in September 2009.
Device and technical details
The GuideLiner™ catheter is a coaxial guide extension with the convenience of rapid exchange. In difficult and challenging interventions guide catheters have a tendency to back out of the artery whereas the GuideLiner™ allows guide extension into the vessel for deep seating. This simplified mother and child technique is useful in challenging interventions and for rapid exchange.
It is composed of a flexible 20 cm straight guide extension for deep seating, connected to a stainless steel push tube with a “collar” which can be deployed through the existing Y-adapter for rapid exchange delivery (Figure 1). Unlike the Heartrail catheter, the GuideLiner™ does not increase the overall guiding catheter length or require a second haemostatic valve, and due to its monorail design is simpler to use than the Heartrail. The GuideLiner™ can be delivered through standard guide catheters, resulting in an inner diameter that is approximately one French size smaller than the guide. The GuideLiner™ is currently available in three sizes: 5-in-6 (0.056” internal diameter), 6-in-7 (0.062” internal diameter) and 7-in-8 (0.071” internal diameter).
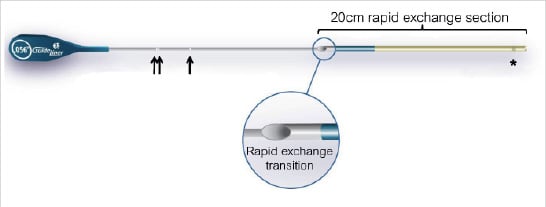
Figure 1. The GuideLiner™ catheter. This consists of a flexible 20 cm straight guide extension connected to a stainless steel push tube. * radiopaque marker 2.66 mm from tip. Arrows: white positioning markers at 94 cm (single arrow) and 105 cm (double arrows).
The extension is 20 cm long, but a maximum extension of only 10 cm is recommended and has a silicon coating for lubricity. The extension section is a component built tube composed of an inner polytetrafluoroethylene (PTFE: Teflon) liner, a middle stainless steel coil (which provides maximum flexibility while retaining radial strength) and an outer polyether block amide (Pebax) polymer extrusion (same material as a guide catheter, and does not soften at body temperature).
There is a radio-opaque marker located 0.105” (2.66 mm) from the tip (Figure 1). The guide extension is connected to the push tube with a “collar”: guidewires, balloons and stents enter the collar within the guide catheter (Figures 1 and 2). The delivery through the guide is designed to be tight in order to prevent slippage within the guide catheter. There are white positioning markers on the push tube at 95 cm (single) and 105 cm (double) to assist in placement through the guide (Figure 1).
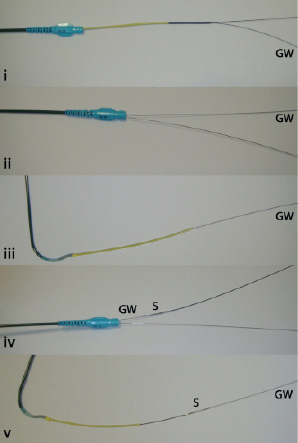
Figure 2. Insertion of the GuideLiner™. The monorail GuideLiner catheter is inserted into a guiding catheter over a guidewire (GW) (i). Once advanced into the guiding catheter, the GuideLiner push tube can be advanced whilst holding the GW in place (ii). The GuideLiner can be advanced up to 10 cm beyond the guiding catheter tip (iii). Balloons or stents (S) can be advanced along the guidewire (iv), through the GuideLiner to the target lesion (v).
Indications for use
1. Deep seating for added back-up guide support in challenging coronary cases to facilitate device delivery.
2. Coaxial alignment when irregular coronary ostium take-off prevents guide placement.
Use of the GuideLiner™ catheter is contraindicated in vessels with less than a 2.5 mm diameter.
Tips and tricks for optimal performance
1. The GuideLiner™ should be inserted into the guide catheter over a 0.014” primary guidewire to a maximum of 10 cm beyond the guide tip under fluoroscopy and in no case more than 20 cm to prevent the metal collar from exiting the guiding catheter.
2. On initial insertion of the GuideLiner™, the flat push-rod should be oriented in a lateral position within the guiding catheter and should be advanced within the guiding catheter without rotation to avoid wrapping the guidewire around it.
3. Deep seating of the GuideLiner™ in the coronary artery can be facilitated by using an un-inflated balloon catheter over the primary wire into distal vessel – if necessary this can then be inflated at the target lesion to act as an anchor, followed by gentle advancement of the GuideLiner™.
4. Stents should be advanced over the primary guidewire through the GuideLiner™ as secondary wires may wrap around the GuideLiner™ push tube, obstructing stent insertion.
5. In case of resistance while inserting a guidewire or stent through the GuideLiner™, the location of the wire or stent in relationship to the metal collar of the GuideLiner™ should be checked and the stent inspected for signs of damage prior to re-advancement. To correct any resistance that occurs at (or proximal to) the collar:
a. Ensure the combination of the wire and stent is compatible with the internal diameter of the GuideLiner™.
b. If a secondary wire is in use, check for wire wrapping of the secondary wire around the GuideLiner™. If so, consider either pulling back the secondary wire or re-advancing it, or if the primary wire (placed before GuideLiner™ insertion) is still in place consider advancing the stent over the primary wire.
c. If a stent continues to encounter resistance at the metal collar, pull the stent and guidewire back together 3-5 cm and try re-advancing the stent and guidewire together through the metal collar. If resistance is again encountered, check the stent for signs of damage and either choose a lower profile stent or change the guidewire.
Clinical experience
A 74 year-old patient with previous coronary artery bypass grafting in 2003 was admitted with a non–ST elevation myocardial infarction (NSTEMI) and inferolateral ST segment changes. His angiogram showed a moderate lesion in the proximal left anterior descending artery (LAD) and a tight stenosis in the circumflex ostium. The graft to the LAD was occluded but there was a patent jump graft to an obtuse marginal and posterior descending artery. The right coronary artery (RCA) was tortuous and calcified with tight stenoses in the proximal and mid vessel (Figures 3a and 3b). Percutaneous intervention to the native RCA was performed transfemorally using a 6 Fr sheath inserted in the right femoral artery. Initially a Hockey stick guiding catheter was used which was changed to an Amplatz Left (AL) 1 guide for better engagement. The RCA was then wired using a BMW wire (Abbott Vascular, Redwood City, CA, USA ) and pre-dilated with a 2.5 x 15 Maverick balloon (Boston Scientific, Natick, MA, USA) (Figures 3c and 3d). However, due to a combination of calcification and tortuosity, a stent could not be delivered. After further dilation with a 3.0 x 15 mm Maverick balloon, it was still impossible to advance a stent. Therefore, a GuideLiner™ catheter was deployed through the AL1 guide and advanced into the mid RCA to aid stent delivery (Figure 3e). This enabled the easy deployment of four overlapping drug-eluting stents from the mid-vessel to the ostium (3.5 x 15 mm, 3.5 x 18 mm, 3.5 x 23 mm and 3.5 x 8 mm; all Promus; Boston Scientific, Natick, MA, USA). The overlaps were post-dilated with a 3.5 x 8 mm non-compliant balloon (Quantum Maverick; Boston Scientific, Natick, MA, USA) whilst the ostium of RCA was post-dilated and flared with a 4 x 8 mm non-compliant balloon (Quantum Maverick; Boston Scientific, Natick, MA, USA). A good angiographic result was achieved (Figure 3f). The patient was discharged the following day with no complications.
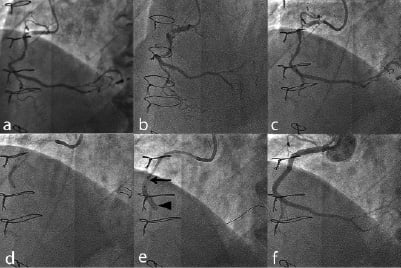
Figure 3. Clinical use of the GuideLiner™. (a) and (b) Diagnostic angiogram of the right coronary artery. (c) Using an Amplatz Left 1 guide, the lesions were crossed with a BMW wire. (d) The lesions were pre-dilated but a stent could not be advanced. (e) The GuideLiner™ (arrow) was advanced up to the lesion to allow deployment of the stent (arrowhead). (f) Final angiographic result.
Discussion
In this case, stent delivery was impossible despite the use of a highly supportive guiding catheter. By using the GuideLiner™, the stent was deployed easily and successfully because of the extra-back up support and deep intubation without any displacement of the guide catheter or the wire or any vessel trauma.
The GuideLiner™ provides a new alternative for performing complex interventions. Benefits include:
1. Deep seating with a straight, highly flexible guide extension.
– Unlike deep intubation of a guiding catheter, there is no primary curve to potentially damage and dissect the vessel.
– Coil backbone provides superior flexibility while retaining radial strength.
2. The device only reduces the lumen by approximately one French size, so almost all devices will still fit through a 6 Fr GuideLiner™ (internal diameter 0.056”).
3. Rapid exchange aids deployment through the existing haemostatic valve without extending the guiding catheter length, and so does not limit the usable length of balloons and wires.

