Abstract
Aims: The Terumo Heartrail™ catheter (Terumo Corp., Tokyo, Japan) allows extra deep catheter intubation of coronary vessels and has been shown to be useful in CTO lesions. The aim of this study is to assess the safety and efficacy of using the Heartrail II catheter as a distal stent delivery system in PCI following failure of conventional techniques.
Methods and results: We prospectively identified cases performed over a 15-month period in which a Heartrail catheter was used to facilitate stent delivery following failure of conventional techniques. Stent delivery using the Heartrail catheter was performed in 35 cases and was successful in 31 cases. Success rates of 100% in grafts, 95% in RCA, 80% in LAD and 60% in circumflex cases were recorded respectively. Successful stent delivery was associated with intubation depth, with 29/29 succeeding when the intubation depth was > 2 cm and failure in 4/5 cases when the intubation depth ≤ 2 cm. There were no complications related to deep intubation of the catheter.
Conclusions: Use of the Heartrail catheter is safe and highly effective for aiding stent delivery across proximal obstructions in both left and right coronary systems. The small number of unsuccessful cases were related to inability of the catheter to traverse stenotic proximal obstructions within 2 cm of the RCA and LCA origins.
Introduction
Several techniques have been proposed to aid distal stent delivery including vessel preparation using balloon angioplasty and rotablation, vessel straightening using support and buddy wires and increasing back up support by deep intubation, change in guide catheter or anchor techniques1-6. Despite the use of many of these techniques, as well as advancements in stent design, failure to deliver a stent into the target lesion remains a common cause of procedural failure.
The Terumo ‘five-in-six’ system, also called ‘mother and child’, involves insertion of a flexible tipped extra length 120 cm 5 Fr guiding catheter (Terumo, Heartrail II catheter; Terumo Corp., Tokyo, Japan) through a standard 100 cm 6 Fr guiding catheter (the ‘mother’ catheter) so that its tip extends into the vessel allowing extra deep intubation and hence increased backup support. Use of this system has been shown to be useful in the treatment of total chronic occlusion cases where such increased backup support is important. In a previous preliminary report, we described a novel use of the Terumo Heartrail II catheter to achieve atraumatic extra deep intubation to aid distal stent delivery following failure of conventional techniques, without disturbing guide or wire position in four cases involving RCA and graft interventions7. We had not previously described use of this technique in the left coronary artery, and could not address issues of efficacy, safety or response in particular lesion subsets in this small series. We have therefore prospectively collected data from a larger extended series involving use of this device for stent delivery in 35 consecutive cases and have examined both factors leading to procedural success and possible limitations of this device for stent delivery.
Methods
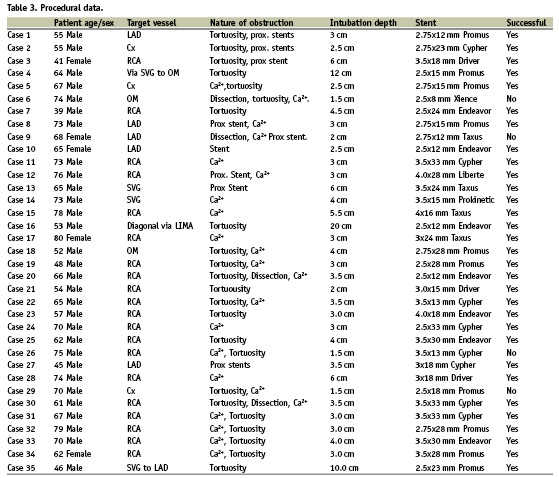
We prospectively analysed 35 successive cases performed at our institution, between July 2007 and October 2008 in which a Heartrail II catheter had been used as a stent delivery device following initial failure using conventional techniques. Case notes and angioplasty films were reviewed by two interventional cardiologists to determine the nature of the proximal obstruction, the total depth of intubation and the success of the Heartrail II catheter to achieve stent delivery. Procedural factors related to success and failure were determined and complications caused by the device were recorded. The Heartrail II catheter was used within 6 Fr (or equivalent) guide catheters in all cases (Cordis Vista Brite Tip, Medtronic Launcher and Asahi Sheathless 6.5 Fr). The haemostatic valve was disconnected from the guide catheter and the heartrail device was passed through the guide catheter into the target vessel either over a coronary wire (two operators), or a wire and a balloon catheter with the balloon un-inflated in the distal vessel (one operator). The haemostatic valve was then re-attached to the end of the Heartrail catheter and the interventional procedure was performed in the usual manner through the haemostatic valve.
Results
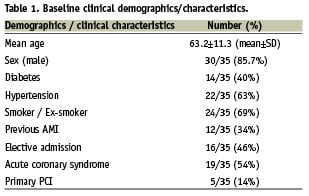
A total of 35 consecutive cases involving use of the Heartrail catheter to aid distal stent delivery where conventional techniques had failed were performed by three operators at our institution over a period of 15 months (3.2% of their total case load). Of the 35 patients in which the Heartrail was used, 30 were male (85.7%) and the mean age was 63.2±11.3 (mean±SD) years old. Demographics data from this cohort of patients is presented in Table 1 and lesion data is presented in Table 2. Procedural data is detailed in Table 3 and angioplasty wire use prior to use of the Heartrail catheter is presented in Table 4.
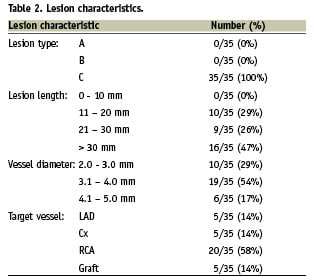

None of the cases involved treatment of chronic total occlusions. The causes of obstruction to stent delivery in these cases were extreme tortuosity (65%) in isolation or in conjunction with either heavy calcification (60%) or previously deployed stents (23%). 16 patients (46 %) were elective admissions and 19 were patients presenting with acute coronary syndromes (ACS), of which five were ST-elevation acute myocardial infarctions transferred for primary PCI. Mean CK (± SEM) 12 hours post-procedure was 150.5±30.2 u/L, 278.6±59.3 u/L and 1654±564.6 u/L in elective admission cases, ACS cases and patients undergoing primary PCI respectively. Twenty of the cases were right coronary artery (RCA) interventions (example in Figure 1), five were left anterior descending (LAD) interventions (example in Figure 2) and five were circumflex (Cx) interventions (14.3%, example in Figure 3). Five cases were grafts interventions involving saphenous vein grafts in four cases and left internal mammary artery intervention in one case.
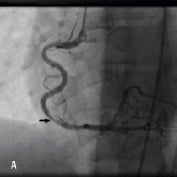
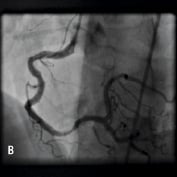
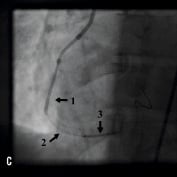
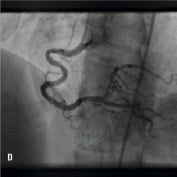
Figure 1. A 41 year old female renal transplant recipient was admitted with an inferior ST-elevation AMI. Angiography revealed a thrombus-laden lesion in a tortuous right coronary artery as illustrated in Figure 1A by arrow. Thrombectomy was performed and the lesion was predilated with a 3.0 mm balloon. The lesion was stented with a 3.5x18 mm Driver stent. Significant plaque shift occurred (Figure 1B). Attempts were made to deliver a second driver 3.5x12 mm Driver stent distally but failed due to failure to bypass the proximal portion of the previously deployed stent despite use of a buddy wire and further balloon dilation. Figure 1C illustrates that deep intubation of the right coronary artery with the Heartrail catheter (arrow 1) up to the proximal portion of the previously deployed stent (arrow 2) allowed passage of a 3.5x12 mm Driver stent distally (arrow 3) hence enabling successful stent delivery. Figure 1D illustrates the final result.
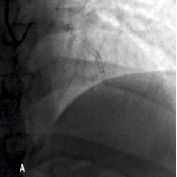
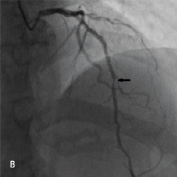
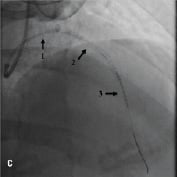
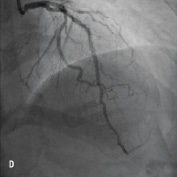
Figure 2. A 45 year old male with a previous history of PCI to a long segment of LAD with 4 Cypher stents (>80 mm) (Figure 2A) was admitted for PCI to a significant lesion just distal to the proximally stented vessel (Figure 2B). Attempts made to deliver a stent distally failed due to significant resistance to stent passage within the stented portion of the vessel despite further balloon dilation. Figure 2C illustrates advancement of the Heartrail catheter (arrow 1) into the stented segment in the LAD (arrow 2) to bypass this proximal point of obstruction thereby enabling distal stent delivery (arrow 3). The final result is illustrated in Figure 2D.
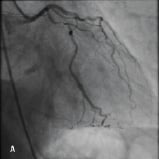
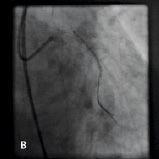
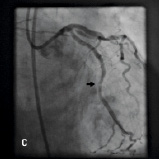
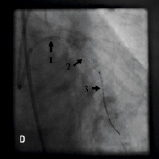
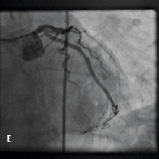
Figure 3. A 55 year old male admitted with an ACS and lateral t-wave changes on ECG underwent cardiac catheterisation which demonstrated a significant lesion in a tortuous, heavily calcified proximal circumflex vessel (Figure 3A). Multiple balloons were used to predilate the lesion and attempts were made to pass a Cypher 2.75x23 mm stent. Due to vessel tortuousity and heavy calcification, stent delivery was not possible hence an extra support buddy wire was used to facilitate stent delivery (Figure 3B). Following deployment of the stent, distal plaque shift was visualised (Figure 3C) as illustrated by black arrow. Further attempts to pass a second Cypher 2.75x23 mm stent to cover this area failed due to significant resistance to stent passage from calcification, tortuosity and a proximally deployed stent despite use of buddy wires. Figure 3D illustrates that a Heartrail catheter (arrow 1) was used to deeply intubate the circumflex up to the proximal stent edge allowing delivery of a Cypher 2.75x23 mm stent (arrow 3) past the proximally deployed stent (arrow 2). Figure 3E illustrates the final result.
The Heartrail catheter was advanced into the vessel either over a coronary wire, or a wire and a balloon catheter with the balloon un-inflated in the distal vessel. In five cases, catheter advancement was aided by anchoring the balloon distally, by inflating it in the target lesion and both pushing gently on the catheter and pulling gently on the balloon. No cases of air embolism were recorded, and importantly, there were no procedural complications related to the use of the catheter such as coronary dissection, coronary perforation or evidence of distal embolisation, bleed back between the «mother» guide catheter and the Heartrail catheter.
Stent delivery was successful with the aid of the Heartrail catheter in 31/34 cases (91.2%). Of the five LAD intervention cases performed, stent delivery was achieved in four cases. In the unsuccessful case (Case 9, Table 3) rotablation and stenting of the LMS (protected), led to extensive dissection in the proximal LAD that was heavily calcified and stenosed. The Heartrail catheter could not be advanced into this segment and, with an intubation depth of 2 cm, stent delivery was not facilitated. Stent delivery was achieved in 3/5 circumflex interventions. In one of the unsuccessful cases (case 6, Table 3) the Heartrail catheter would not advance into an angulated and calcified lesion in the proximal Cx that was dissected following predilation. The intubation depth was 1.5 cm, and stent advancement into this lesion was not facilitated. Similarly, in the second case, the Heartrail catheter could not be advanced into an angulated, heavily calcified ostial circumflex lesion and with an intubation depth of < 2 cm, stent delivery was not facilitated. Stent delivery was successful in 19/20 RCA interventions. In the single unsuccessful case (Case 26, Table 3), the Heartrail catheter could not be advanced into a heavily calcified proximal lesion, and with an intubation depth of 1.5 cm, stent delivery was not facilitated (Figure 4). Stent delivery was successful in 5/5 graft interventions.
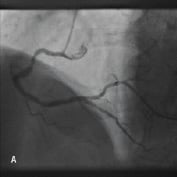
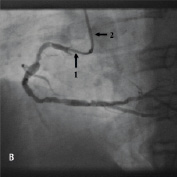
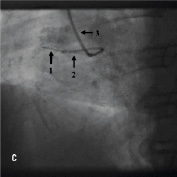
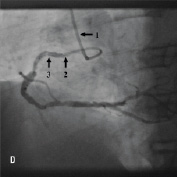
Figure 4. A 75 year old male was admitted for elective PCI to a heavily calcified lesion in the proximal right coronary artery just distal to a tortuous section resembling a shepherds crook (Figure 4A). Extensive ballooning was performed to predilate the lesion and attempts were made to pass a Cypher 3.5x13 mm stent into the proximal lesion which despite the use of buddy wire technique failed. A Heartrail catheter (Figure 4B, Arrow 1) was used to attempt to bypass this proximal obstruction but significant resistance was encountered. Even with the 6 Fr JRF guide catheter (Arrow 2) resting on the contralateral aortic wall which would provide significant back-up support we were unable to bypass the proximal lesion with the Heartrail catheter (Figure 4C Arrow 2) or deliver the stent (Figure 4C arrow 1) distally. Figure 4D illustrates the vessel filled with contrast with the guide catheter (arrow 1), Heartrail catheter (arrow 2) and stent (arrow 3) position shown. The procedure was abandoned following failure of stent delivery.
In the cases in which distal stent delivery using the Heartrail catheter failed, the main problem appeared to be due to failure of the catheter to bypass very proximal points of obstruction such as a dissection flap, as in cases 6 and 9; and severe proximal calcification and tortuosity in cases 26 and 29. Difficulties appeared to be more frequently associated with circumflex intubations (failure in 2/5 cases), possibly related to difficulties associated with acute angulation into the circumflex from the LMS. In all cases that failed, the depth of intubation of the Heartrail catheter was <2 cm (mean 1.6 cm), whilst in successful cases mean intubation depth was 4.6 cm. Indeed, of the five cases performed where depth of intubation of the Heartrail catheter was <2 cm, only one case was successful (20%), whereas 29/29 cases were successful when intubation depths of greater than 2 cm were achieved.
Discussion
In this consecutive series prior attempts at stent delivery had failed despite the use of buddy or extra support wires by highly experienced interventionalists. Successful stent delivery was achieved in these cases using the Heartrail catheter in almost 90% of cases. The device was deployed within a range of standard 6 Fr (or equivalent) guide catheters without displacing either the guide catheter or coronary wires, and was not associated with vessel trauma in any case despite extra deep intubation into extensively diseased proximal arterial segments. This shows that this device is simple to use, highly effective and safe, and enables procedures to be performed successfully that would have failed without the use of this device.
The operators involved in this study used the Heartrail catheter to aid stent delivery in approximately one in 30 procedures. Failure to deliver a stent in these cases occurred despite lesion preparation using pre-dilatation occured in all cases, and the use of extra support and / or buddy wires in 31/35 cases. In three cases, use of the Heartrail catheter achieved stent delivery with subsequent procedural success after a previous procedure was abandoned due to failure to deliver stents using conventional techniques. Unsuccessful stent delivery, despite use of the Heartrail catheter, was related in all cases to very proximal obstructions that could not be crossed with the catheter. Proportionally, this occurred most frequently for circumflex interventions, with failure of the catheter to traverse angulated lesions at the bifurcation of the LMS into the circumflex. Procedural success was 100% when the intubation depth exceeded 2 cm.
In our initial preliminary report we did not include left coronary artery (LCA) interventions. We had concerns that using the catheter could lead to vessel wall trauma in the setting of acute angulation or significant proximal disease in LAD, circumflex or left main stem. In this study, we have now shown that the Heartrail catheter is a safe device with no vessel wall trauma or embolisation detected in any case, including 10 LCA procedures. This is despite use of the catheter in highly tortuous heavily calcified vessels with intubation depths of up to 6 cm in native coronary arteries. This is likely to be due to the soft and flexible tip of the Heartrail catheter in comparison to using conventional guide catheters. Pressure damping of the catheter was occasionally observed when the catheter was advanced into stenotic lesions, but this was less frequent than anticipated due to the small diameter of the catheter (5 Fr rather than 6 Fr), and the use of the catheter to cross proximal obstructions that in most cases were not stenotic. As with the use of all 5 Fr catheters, care withdrawing and introducing balloons and stents was required to avoid sucking air into the catheter which could then be injected distally.
The Heartrail catheter is designed to fit within 6 Fr or larger catheters with an internal lumen of at least 0.071 inches. In this series we also used the catheter without difficulty within Cordis Vista Brite Tip 6 Fr guide catheters (Cordis, Johnson & Johnson, Warren, NJ, USA) and Asahi Sheathless 6.5 Fr guide catheters (ASAHI Intecc, Aichi, Japan) which have internal lumens of 0.070 inches. The Heartrail catheter has a larger internal lumen than other 5 Fr catheters at 0.059 inches. We were able to advance drug eluting stents of up to 4 mm diameter within this catheter without resistance (Table 3) and were able to advance smaller diameter stents with 2 support wires in situ.
Extra deep intubation with the Heartrail catheter facilitates stent delivery by traversing proximal points of obstruction and by increasing back-up support. Prior to deployment, stents are rigid and may develop significant friction with the vessel wall when calcification or a deployed stent strut are present at points of vessel angulation, even in the absence of significant luminal narrowing. The Heartrail catheter has a soft and very flexible tip that may cross such points of obstruction without resistance. When this has been achieved, a stent may be delivered through the Heartrail catheter without resistance. The increase in backup support associated with deep intubation is well documented. In an experimental arterial model, Takahashi et al8 illustrated that backup support was increased with depth of intubation so that at 5 mm depth of intubation backup support was almost 2 fold greater than with a 6 Fr guide catheter alone, and that further deeper intubation was associated with even greater backup support. Greater backup support allows more force to be applied to advance a stent distally thereby helping to overcome friction. We postulate that when the intubation depth is less than 2 cm neither of the mechanisms may be activated with the catheter adding little to stent delivery. Conversely, when significant intubation depth is achieved, it is likely that both mechanisms contribute to successful stent delivery in most cases.
Use of this system has been shown to be useful in the treatment of total chronic occlusion cases8-10 where increased backup support is important for wire penetration into the CTO and subsequent balloon and stent advancement. This series describes its role as a stent delivery device and does not include CTO cases. Conventional techniques for facilitating stent delivery include use of buddy wires and support wires to reduce tortuosity1,2, use of rotablation / balloon dilation in heavily calcified vessels to reduce friction3,4, use of smaller sized stents11, or increased backup support by deep intubation of the guide catheter, anchor balloon techniques or exchanging to a larger guide catheter such as a 7 Fr or 8 Fr system5,6,12. Recently, the use of topical lubricants applied locally onto the stent to reduce friction with the vessel wall and allow for distal delivery has been described13. The simplest of these techniques, namely repeat balloon dilation and the use of support and buddy wires can be used either prior to, or in conjunction with the Heartrail catheter. The more advanced techniques such as anchor balloon prior to stenting, rotablation and use of a stiffer guide catheter, frequently require change of either the guide catheter (from a standard 6 Fr catheter) or change of the coronary wire. Operators may be reluctant to do this once guide catheter and coronary wires are in place and a complex lesion has been predilated. One of the principal advantages of this technique is that the position of the ‘mother’ guide and coronary wire are not disturbed, and the technique can be used as required if difficulties concerning stent delivery are encountered. Difficulties with stent delivery occur in a significant proportion of cases, for instance in the recent REALITY trial, nearly 5% of lesions could not be successfully treated with the assigned drug-eluting stent14. These difficulties are likely to increase as interventionalists treat more calcific and tortuous vessels in older patients.
Potential improvements in Heartrail catheter design, which would optimise its use to bypass points of obstruction, include a lower crossing profile and a hydrophilic coating particularly at the tip of the catheter. A smaller sized catheter, for example 4 Fr would make the catheter more deliverable and potentially reduce the possibility for complications further, although at the expense of a reduced backup support and limitation of the diameter of stent that could be accommodated.
In conclusion, we have demonstrated that use of the Heartrail catheter is both a safe and effective means for distal stent delivery across proximal obstructions in both left and right coronary systems and coronary grafts. Its principal limitation is in vessels with highly stenotic proximal obstructions within 2 cm of the RCA and LCA origins through which the catheter cannot pass. This catheter can be recommended, not only as a bail-out device when all other techniques have failed, but should also be considered early in a procedure when difficulties with stent delivery are anticipated. This is supported by its ease of use, high procedural success, and low incidence of complications.

