Abstract
Aims: Applying the Magnetic Navigation System (MNS) to manage chronic total occlusions (CTOs). The MNS precisely directs a magnetised guidewire in vivo through two permanent external magnets.
Methods and results: The first 43 consecutive MNS treated CTOs were retrospectively evaluated. Computed tomography coronary angiography (CTCA) co-integration with the MNS provided a virtual road map through the occlusion. Unsuccessful MNS cases were managed with bailout conventional guidewire techniques. Experienced CTO and MNS operators had unrestricted access to CTO devices and equipments. The MNS crossing success increased from 40% to 56% over 52 months and averaged 44.2% (19/43 patients). In 58.3% (14/24) of failed MNS cases the conventional wire approach was successful, giving an overall procedural success rate of 76.6%. Of those conventionally treated, two patients required pericardiocentesis. On average, 1.8±0.9 stents (lengths 44.7±21.4 mm and diameter 2.8±0.4 mm) were implanted. Procedural times were lengthy (125.0±35.3 min) requiring high fluoroscopy dosage (11980.2±6457.9 Gy/cm2) and contrast media usage (388.8±170.2 ml). Operators persevered less with magnetic wires (20.9±12.4 min vs. 27.7±24.4 min), and preferentially used the least stiff wire as first choice (53.5%). CTCA co-integration did not influence procedural outcome. As with conventional wires, higher magnetic wire successes occurred in low calcified lesions, those with a central stump and without bridging collaterals.
Conclusions: In unselected CTOs, the magnetic wires are safe and feasible. Current modest success rates with a high procedural bailout rate implicate the need for improved magnetic guidewire technology comparable to available sophisticated conventional CTO wires. Randomised studies are needed to clarify the value of magnetic guided recanalisation.
Introduction
Chronic total occlusions (CTO) are encountered in over 25% of patients undergoing percutaneous coronary interventions (PCI)1,2. Their importance stems from the fact that treatment relieves symptoms, increases exercise tolerance and improves left ventricular function3. Moreover, recanalisation reduces the need for coronary artery bypass surgery and leads to better long-term survival4. But despite a high proportion of CTOs having recanalised lumen, crossing the occlusion remains a challenge5. This is because factors such as calcification, the occlusion length, age, the nature of the stump, the presence of collaterals together with the immediate anatomical environment of the CTO can all influence the outcome6. These vessel characteristics together with ability of the skilled operator in responding to tactile feedback prior to advancing sophisticated conventional guidewires can be crucial to the overall procedural success7.
The magnetic navigation system (MNS) has the ability to steer through a vessel by changing the tip of a magnetically enabled guidewire without the need to remove it from the patient8. It has been reported that this ability can aid the search for micro-channels to effectively negotiate through the occlusion9. Moreover, by co-integrating data obtained from computed tomography coronary angiography (CTCA) into the MNS software a navigational pathway can be derived from the inherent radio-opacity of the occluded segment tissue that is non delineated by contrast media to be used in the guidewire navigation10. In this study we report on the feasibility and safety of this novel approach and our experience on the factors that influenced the procedural outcome with the MNS.
Material and methods
Study patients
In a 52 month study period from February 2004 to May 2008, forty-three consecutive patients presenting with symptomatic coronary artery disease due to a chronically occluded vessel were managed with the MNS. The selection criteria for a chronic occlusion was defined as either an occlusion on angiography without antegrade filling of the distal vessel other than via collaterals or minimal antegrade flow (TIMI flow 0 or 1). All patients included had a native vessel occlusion estimated to be at least of one month’s duration, defined according to previously published studies and based on either a history of sudden chest pain, a previous acute myocardial infarction in the same target vessel territory, or the time between the diagnosis made on coronary angiography and PCI6,7. The type of CTO was either related to de novo stenoses or in-stent restenosis. The protocol was locally approved and all patients gave written informed consent. In total, five cardiologists experienced in CTO and trained with the MNS had unrestricted access to the choice of either magnetic or conventional guidewires. In addition, one experienced MNS technician was required to operate the navigational software. Patients were excluded if they were haemodynamically unstable or perceived contraindications for magnetic-assisted intervention (MAI) such as having a pacemaker.
CTCA acquisition
CTCA was performed on 64-slice CT (Sensation 64, Siemens AG, Forchheim, Germany) using a bolus of 100 ml contrast fluid (iomeprol, Iomeron® 400; Bracco, Milan, Italy) intravenously injected at a rate of 5 ml/sec. ECG-gating dataset were reconstructed during the mid-to-end diastolic phase (300 to 450 ms before the next R-wave or 60% to 70% of the R-R interval). The coronary artery was automatically extracted off-line using dedicated post processing software (Leonardo VB30A; Siemens) and incorporated directly in the MNS navigational software called the Navigant®.
The Magnetic Navigation System
The Niobe® II Magnetic Navigation System (MNS) (Stereotaxis, St. Louis, MO, USA) has been previously described8. Briefly two permanent magnets generate a field (0.08Tesla) that can be focused to follow navigational vectors created in Navigant®. The vectors are derived through various navigational modes based on 2-dimensional or 3-dimensional information. In addition, they can be automatically updated or manually controlled via a touch sensitive monitor situated at arm’s length from the operating table.
Navigational modes used in this study
THE CLOCKFACE NAVIGATION
In the clockface navigation mode vectors are orientated through a touch sensitive monitor that mimics the dial of a clock (Figure 1A).
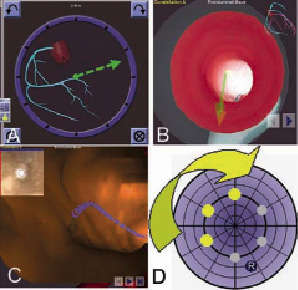
Figure 1: A. Clockface navigation mode vectors are orientated by through a touch sensitive monitor; B. CardiOpB® virtual 3DRC vessel showing fly-through endoluminal view for navigation; C. Extracted CTCA virtual fly-through endoluminal view with MPR slice in the top left corner; D. The bull’s eye navigational mode demonstrating the ability to reorientate the wire tip in a systematic manner.
The vectors move in the X-Y axis and there is an option for a third dimension (Z axis). Full 360º omni rotation of the magnetically enabled wire is therefore possible but to be effective a spatial understanding of coronary anatomy is required.
THREE-DIMENSIONAL (3-D) VIRTUAL RECONSTRUCTION (3DRC)
The CardiOpB® (Paieon Medical Inc., Rosh Ha’ayin, Israel) uses two orthogonal x-ray images that are at least 30° apart to create a virtual 3DRC vessel. A navigational path associated with vector created in Navigant® is then overlaid on the live fluoroscopic image after alignment with the tip of a guiding catheter. The system also incorporates a fly-through endoluminal view for navigation (Figure 1B).
THE CO-INTEGRATION OF CTCA
Incorporating the extracted CTCA into Navigant® allows both the mapping and co-registration of the occluded segment. Superimposing the derived navigational pathway on the fluoroscopic images enables navigation to be performed in a similar manner to a 3DRC. But in addition to providing an endoluminal view, the system can simultaneously display the multi-planer reconstructed (MPR) slices through the occlusion thus allowing the operator to visualise upfront the morphology of the occlusion (Figure 1C).
THE BULL’SEYE VIEW
Regardless of the 2D or 3D navigational mode, the Bull’s Eye model can be additionally employed to search for microchannels in a systematic way. Here the wire tip can be automatically or manually re-orientated in a systematic fashion to mimic the contours of a dartboard or a bull’s eye (Figure 1D).
Special CTO techniques
In this study over-the-wire balloons (OTW) provided additional back up for the guidewires. It also facilitated the rapid exchange of various guidewires though its lumen without losing the original wire position. Subsequent removal of the OTW required inflation of an indeflator containing saline to roughly 16 atmospheres connected to the lumen.
Employing two guidewires to search for the true lumen was used in the parallel wire technique: one that has entered the vessel subintimally and left in this position with another usually a stiffer wire used to find the true lumen from a different entry point. In a somewhat similar technique, the see-saw technique used two guidewires, but instead of leaving one of them in the false lumen, both are used sequentially to re-enter in the true lumen11.
The guidewires
The most common MNS wires used in this study were the first generation Cronus™ and second-generation Titan™ Soft Support coronary guidewire (Stereotaxis, St. Louis, MO, USA). They are both 180 cm long hydrophilic coated, 0.014 in wires with a flexible 2 cm distal coiled tip having a 2 mm or 3 mm long gold cup neodynium magnet attached. In one case the newest guidewire, the Pegasus™ was used. This is similar to the Titan™ Soft Support coronary guidewire except the distal 30 cm of the stainless steel distal shaft is replaced by nitinol. All three generations are available in the stiffer Assert™ wire ranges. The conventional wires used were PT Graphix™ Intermediate, Choice™ PT Floppy (Boston Scientific Corp. Miami, FL, USA); Pilot™ 50, 150, Whisper™ and high torque BMW (Abbott Vascular Devices, Redwood CA, USA); Miracle bros™ 3G, 4.5G, 6G, 12G, Fielder and Confianza (ASAHI INTEC, Co., Nagoya, Japan); Crosswire NT (Terumo Inc., Tokyo, Japan).
Statistics
Continuous variables are presented as means and standard deviation. T-test and one-way ANOVA (Bonferoni) were used to determine if there were significant differences in the procedural time and fluoroscopic times. Analyses were performed using SPSS 11.5 for Windows (SPSS, Chicago, IL, USA). P value <0.05 was considered statistically significant.
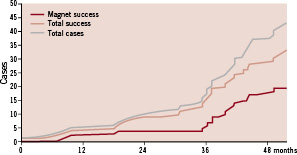
Figure 2. Cumulative curve of CTO success with the MNS with respect to the total success and cases.
Result
Demographics and CTO characteristics
The baseline characteristics of the 43 patients showed that the mean age of patients were 67.1±9.8 years and the majority were male (76.7%). One fifth were diabetics, of which 14% were insulin dependent. Other common cardiovascular risk factors were hypertension (60.5%), hyperlipidaemia (76.7%), a positive family history of coronary disease (27.9%) and prior smoking history (48.8%). A significant number of patients had a previous PCI (41.7%) or coronary artery bypass surgery (14.0%) at presentation. On diagnostic angiographic images there was roughly equal distribution of one (34.9%), two (39.5%) and three vessel (25.6%) disease.
The majority of patients (93.0%) underwent a first attempt to revascularise the CTO. Most CTOs were de novo lesions (95.3%) frequently involving the left anterior descending artery (LAD, 32.6%) and to a lesser extent, the circumflex coronary artery (LCX, 25.5%), the right coronary artery (RCA, 18.6%), an intermediate or an obtuse marginal branch (14%) and the diagonal branch of the LAD (9.3%). In line with published data, stump morphology was found to be equally distributed12 (Table 1).
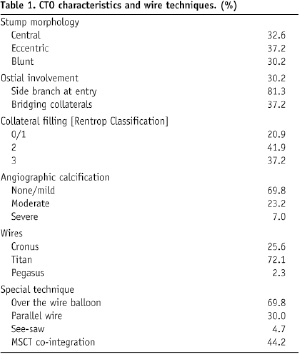
Over three-quarters of the cases had significant contralateral collateralisation. Almost one third had significant vessel calcification on angiography. In addition to the occluded vessels, one third of the patients had a second single vessel treated at the indexed procedure and two other vessels treated in 9.3% of the cases.
Success of the Magnetic Navigation System in crossing the occlusion
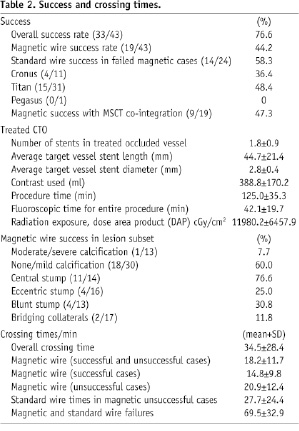
The overall crossing success using the full armamentarium of all possible choices of magnetically enabled wires and navigational modes was 44.2% (19/43patients). Bail-out conventional wires were successful in 14 of the failed 24 magnetic cases (58.3%) giving an overall procedural success of 76.6%. In the majority of cases the second generation Titan™ wires were used (72.1%). These wires had a greater overall crossing success over the first generation Cronus™ range (48.4% vs. 36.4%). A significant proportion (44.2%) of patients had CTCA co-integration into Navigant® to create vectors along a navigational path through the occlusion. Surprisingly, the overall success in patients where the CTCA was co-integrated and navigated upon was nearly identical to those managed without CTCA co-integration (14/19, 73.6% vs. 18/24, 75%, respectively). Moreover, CTCA co-integration only marginally improved the magnetic wire success (9/19, 47.3%) when compared to the overall success achieved with this system (19/43, 44.2%).
On average 1.8±0.9 stents with lengths 44.7±21.4 mm and diameters 2.8±0.4 mm were used to recanalise the vessels. The average procedural time was 125.0±35.3 min with an overall contrast media usage of 388.8±170.2 ml. Additionally, patients were exposed to lengthy radiation times 42.1±19.7 min and dose of radiation exposure 11980.2±6457.9 cGy/cm2. The amount of DAP exposure therefore was similar to that previously reported in an assessment of 165 CTOs7. We estimate a mean effective radiation dose for CTCA in CTO to be 22.4 mSv as this amount was previously reported in a similar patient population at our institution7. At routine clinical follow-up our patients did not report and radiation injury during or after the procedure. The overall procedural complication was low (4.6%) with no complications observed with the magnetic wires. Two patients required pericardial drainage insertions following bail out conventional wire procedures and ballooning. One patient proceeded to emergency surgery following an extra-vascular balloon dilatation.
Analysis of Magnetic Navigation System performance in crossing the occlusion
Following the instalment of the MNS in 2004 the feasibility of managing CTOs was performed initially in five patients using the first generation wires (Cronus™) and this wire had a success rate of only 40%. A low patient recruitment was observed in the subsequent two years but this improved following the integration of the CTCA extracted coronary artery into the Navigant®. In combination with the second generation wires (Titan™) more patients were treated (53%) and the success rate improved to 56%.
In terms of the wire performances, the average time for wiring the CTO whether successful or not regardless of the technique (magnetic or bail-out) was 34.5±28.4 min. Within this period the magnetic wire was used for average of 18.2±11.7 min. In the 19 successful magnetic cases the average crossing time was 14.8±9.8 min and in the 24 failed cases the average trial time was 20.9±12.4 min. Wiring time successes with the bailout conventional wire techniques in the 14 failed magnetic cases was 27.7±24.4 min and revealed no significant difference to the time taken with the unsuccessful MNS (p=0.22). In 10 cases (23.3%) in which both guiding techniques failed the total wiring time was 69.5±32.9 min. Analysis of these cases revealed that the majority (six cases) was due to vessel dissections after several trials with the conventional wire. The inability to penetrate the cap or re-enter the true lumen distally each accounted for the two failed cases. Analysis of the lesion characteristics revealed that the magnetic wires were more successful if the stump was central (76.6%) and if there were low levels (none/mild) of calcification (60.0%). As recognised with conventional wires limited magnetic wire successes were observed in vessels having moderate/severe calcification (7.7%) or in cases were there was angiographically bridging collaterals (11.8%), Table 2.
Assessment of wire usage
In total, twenty different types of guidewires (seven magnetic and 13 conventional) were used in this study (Table 3).
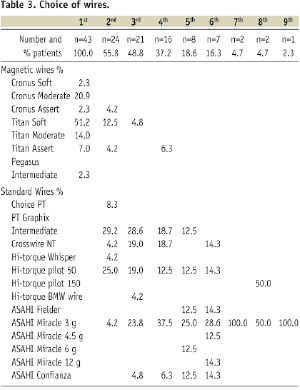
Moreover in 70% of the cases, wires were supported with OTW balloons. Overall operators were less inclined to use stiffer magnetic wires early in the procedure. They also gave up similarly weighted magnetic wire (the 3 gram Assert range) relatively earlier (fourth choice) in favour of the 3 gram Miracle wires, which was employed as far as a ninth choice. Amongst the first generation magnetic wires the Cronus™ Moderate was the first choice (20.9%) with stiffer Cronus™ 3 gram Assert guidewires used as a second choice in one case only (Table 3). Softer second generation wires (Titan™ Soft Support) were used as first choice in over fifty percent of cases and the stiffer Titan™ Moderate or Asset wires were used as the first choice only in a relatively small number of procedures (21%). Comparable to the MNS approach, softer conventional wires (PT choice, Hi Torque Whisper, Hi Torque BMW) were preferentially used as the first choice (36.5%) in bailout MNS cases. More hydrophilic (PT Graphic Intermediate and Crosswire NT) and stiffer wires (Miracle 3 gram and Confianza) were more often used as a third choice (47.6% and 28.6%, respectively). The prevalence of the stiffer conventional wires increased over time.
Discussion
Chronic total occlusions are both challenging and prognostically significant10. Patients are often subjected to lengthy procedures, high volumes of contrast media impacting on renal function, and high levels of radiation exposure13,14. Moreover, the high procedural cost can have repercussions on budgets and resources15. To try and improve outcome the magnetic navigation system is being evaluated in this lesion subset10. There is data to support its use in other complex vascular anatomies16 leading to reduction in contrast media usage and also in selected failed conventional guidewire cases17. But thus far, only two registries provide data on CTOs. In a small population N=30, three out of the five failed magnetically cases18 and in a larger study (N=350) 25 out of the 35 failed magnetic crossings were CTOs19.
This is the first series specifically detailing the success of CTOs in a leading centre since the MNS installation in 20049. It demonstrates modest success, but shows that the MNS can be safely used in unselected occlusions. In addition there is a learning curve, as was seen with other magnetic coronary and non-coronary interventions19-21. However, despite this, the MNS overall success rate was lower than that historically reported from our centre6 necessitating bailout conventional wire strategy to improve procedural success. Complications were restricted to this bailout group since in general operators preferentially used softer magnetic wires and gave up much earlier in preference to the traditionally stiffer dedicated CTO standard wires. Lack of perseverance with the magnetic wires could be due to its novelty and lesser familiarity in this lesion subset. But although the conventional 2-3 mm magnetised tip is effective for patent vessels such a profile may not be ideally suited for CTOs where angulated microchannels negotiation will be limited through the straight blunt tip22. Furthermore the transition of the magnetic tip to the shaft lends itself to “buckling” when “push force” is applied to the wire resulting in operators resorting to the conventional wires having smoother tip transition with the shaft so as not to compromise the applied force. Supporting the magnetic wire with an OTW balloon does not solve this crucial limitation because although the shaft is supported, the advancement of the balloon to support the tip incapacitates the steering ability and furthermore changes the profile of the wire to such an extent that the tactile responsiveness of the tip is completely lost.
These results suggests that for the MNS to be effective in the all CTOs and not restricted to those having central stumps, low calcium or collateralisation of a completely different wire with a different procedural strategy is required. Non-steerable radiofrequency and laser-guided wires have been previously used to manage CTOs with limited success10. Adding steering ability to such a device can potentially improve the outcome in managing CTOs. But in order to be safe and effective, precision mapping of the occluded segment with real time feedback positioning of the device within a fraction of a millimetre is required. A static navigational pathway through the occlusion overlaid on the fluoroscopy images does not seem to influence the success in patients with current magnetic wires. Stereotaxis recognises this and is currently developing a magnetically enabled RF ablating wire to be used with the CTCA8. But to be effective this CTCA roadmap needs to spontaneously updated with the changing vessel anatomy of the beating heart (dynamic road mapping) to optimally position the device prior to delivering the energy. Moreover, non-cardiac factors such as patient movement and respiratory/chest wall motion will need to be adequately compensated and overcome which is by no means an easy task.
Limitations
The level of experiences with the two strategies may have influenced the period of time operators were willing to persevere with each system. There was no written or universal approach to deal with the various complexities encountered. The study was not powered to assess the influence of guiding catheters, which could have had an influence on the procedural outcome. Contrast media usage and fluoroscopic times were assessed for the whole procedure and this included the treatment of non target vessels. The age of the occlusion could not be precisely determined, relying primarily on the patient’s clinical history and previous angiographic films. This was a single-centre’s experience in a university specialist tertiary referral unit and the case mix might be different to that seen elsewhere.
Conclusion
In unselected occluded vessels the use of magnetic guiding wire navigation is safe and feasible. Current moderate success rates with a high procedural bailout rate imply the need for improved magnetic guidewire technology comparable to available sophisticated conventional CTO wires. At present CTCA co-integration did not influence procedural outcome. Randomised studies are needed to clarify the value of magnetic guided recanalisation.

