Abstract
Magnetic navigation (MN) can precisely control a percutaneous coronary interventions (PCI) guidewire or a device in three-dimensional space within the body without requiring reshaping of the tip to access vessels or areas of the heart that are often challenging using conventional wires. In this article we review and report on the use of magnetic navigation system in secondary revascularisation of coronary arterial bypass grafts (CABG). MN was successfully used in the secondary revascularisation of failed conventional CABG cases. Retrograde PCI through a LIMA is not only feasible but the wires can manage complex stenoses involving a bifurcation by using 3D reconstruction software. Difficult anatomies such as a hairpin bend as highlighted in this paper found at a saphenous vein graft (SVG) anastomosis can be overcome by co-integrating a CTCA 3D dataset for navigation. Preliminary data supports potential advantages in reduction of contrast media usage, crossing and fluoroscopy times and suggest that larger randomised studies are warranted.
Introduction
The rationale for secondary revascularisation of grafts
Several observational studies have encouraged the use of coronary artery bypass grafting (CABG) in patients with multivessel disease (two-vessel disease and three-vessel disease) including the proximal left anterior descending artery and in those patients with diabetics or having decreased ejection fraction1. This is supported by a data examining twenty-three randomised controlled trials (RCTs) comparing 5,019 patients randomly assigned to PCI and 4,944 patients randomly assigned to CABG2. In this analysis the risk difference for repeat revascularisation with PCI was 24% greater at one year and 33% greater at five years (P<0.001). The absolute rates of repeat revascularisation were 40.1% after PCI with stents and 9.8% after CABG at five years. Recently, SYNTAX has suggested that at patients with SYNTAX scores greater than 32 ideally should be recommended for surgery3. It is likely, therefore, to see a significant increase in secondary revascularisation of surgical patients in the future.
There are few randomised studies assessing PCI in patients with CABG. Results from the Reduction of Restenosis In Saphenous vein grafts with Cypher sirolimus-eluting stent (RRISC) trial – a small, randomised study – demonstrated markedly lower target vessel revascularisation (TVR) rates in saphenous vein graft (SVG) lesions treated with sirolimus-eluting stents (SES), compared with bare metal stents (BMS; 5% and 27%, respectively)4. But the early benefit of reduced target vessel revascularisation disappeared during 3-year follow-up, and SES was associated – ominously – with an increased risk for death5. This was, however, a small study done in patients having early discontinuation of dual antiplatelet therapy. In the Stenting Of Saphenous Vein Grafts (SOS) trial using paclitaxel-eluting stent (Taxus™) in SVG lesions a reduced incidence (9% vs. 51%) of in-stent restenosis after 12 months when compared to a similar bare metal stent6. Despite this early benefit, there remain issues regarding the long-term safety and efficacy of DES in SVG lesions particularly because long-term events frequently result from the progression of other lesions in the vein.
The problem associated with secondary revascularisation of grafts
Beyond one year and particularly >3 years after surgery, atherosclerosis of vein grafts becomes increasingly prevalent. The nature of the plaques is characteristically different than those seen in arteries. In particular, they are often larger compared with plaques in native coronary arteries and are usually soft and friable, rich in necrotic debris, cholesterol crystals, blood elements, and foam cells7. Moreover, they are often associated with thrombus that can cause distal embolisation, inadequate dilatation, and increased restenosis rates. Distal and proximal protecting devices have been shown to reduced embolisation and improve outcome. But, plaque eccentricity and friability together with vascular characteristics such the tortuosity and the acute angulations at the site of the anastomoses to the native vessel can all influence the procedural success7. Uncertainty surrounds the number of cases where this can occur, as authors do not report how frequently patients prepared to receive the device could not because the occlusion could not be traversed due to the vessel angulation or guidewire trauma of the friable plaque resulting in dissection.
The Niobe® Magnetic Navigation System
Magnetic navigation is a rapidly evolving technology in the field of interventional cardiology8. It allows the precise control of a guidewire or a device in three-dimensional space within the body without requiring reshaping of the tip to access vessels or areas of the heart that are often challenging using conventional wires. The concept is not new and was tested more than half a century ago in the peripheral vasculature of animals and in the seventies in man. The modern Niobe® Magnetic Navigation System (MNS) is now well documented with several reviews on the subject8. Briefly, it comprises of two external magnets (0.08 Tesla) that are capable of moving in all planes to generate magnetic vectors that can realign a guidewire having a small magnetic tip. Dedicated software called Navigant® (Stereotaxis, St Louis, MO, USA) controls the magnets by creating a virtual roadmap. Both the magnetic vectors and virtual roadmap are displayed on a real-time fluoroscopy system to guide the operator who uses a sterile touch-screen monitor at the operating table to modify the vectors or the speed at which they are updated (Figure 1). The system is very versatile and caters to the variable experience of different operators. This is possible through the various modes of navigation, which utilises either 2-dimensional or 3-dimensional road maps. In the simplest 2-D mode the operator may choose to use the ‘clock face’ navigation where the vectors are made to point like the dial of a clock. When more complex anatomical navigation is desirable, then the 3-D navigation, which uses a virtually reconstructed vessel from two radiographic images (Figure 1a), or alternatively by directly integrating computed tomographic coronary angiography (CTCA) into the Navigant®, is best suited for optimal navigation.
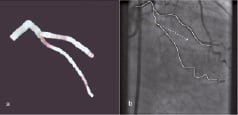
Figure 1. Panel A: shows a virtually reconstructed vessel from 2 angiographic projections using Paeion software. Panel B: shows the virtually created pathway using the vessel centreline, as well as the vector that represents the resulting orientation of the guidewire tip under the applied magnetic field.
The use of the MNS in cardiac electrophysiological studies is now well established. There are fewer randomised studies in the field of interventional cardiology, but amongst them, the MNS has a trend to support its use in crossing complex lesion9. These include tortuous vessels and stented bifurcations where, apart from greater crossing success, it has been shown to reduce both fluoroscopic times and contrast media usage. Moreover, there are several communications demonstrating MNS procedural success in cases that failed using conventional wire steering techniques10. One such case involved a 60 year old man with jump SVG anastomosed to the obtuse marginal branch (OM) and then the right posterior descending coronary artery (RDP)11. Two-months after surgery the graft was occluded, however a section of graft between the OM and the RDP was still fully patent. However, severe lesions at both anastomoses compromised this section and, in addition, there was an adversely acute angle in the stenosis just before the OM anastomoses which was not possible to cross conventionally despite using multiple catheters and wires. Crossing was successful using the MNS, integrating a 3-D reconstruction that provided an endoluminal view with a computed 3-D centreline. This enabled the operator to precisely direct the wire tip with an accuracy that allowed the wire to circumnavigate through the vessel tortuosity equivalent to three cork-screw turns and then enter the adverse acute angulation/stenosis at the OM anastomoses and subsequently to support stent deployment.
The advantages of having an externally steerable wire means that the system can encompass all types of grafts. In particular mammary conduits that can sometimes be difficult to selectively engage with catheters for interventional procedures. By using the magnetic wire to traverse the vessel, selective catheter cannulation can be accomplished by guiding the catheter over the guidewire. As with venous grafts tortuosity, acute bends and difficult anastomoses can be overcome, this is illustrated in Case 1 below.
Initial experiences of using Magnetic Navigation in patients with CABG
In the 12 month period (Oct 2006 - Sept 2007) six CABG patients were enrolled in our institution as part of a randomised (111 patients), all-comers, study comparing the performances of the MNS to conventional wire techniques in crossing flow limiting lesions in patent vessels. The study directly compared the crossing times as ascertained by the wire location at the tip of the guiding catheter to a point beyond the lesion. In addition to the crossing times, both fluoroscopy times and contrast media usage were evaluated. In these six patients, target lesions were identified in three patients having lesions in the native left anterior descendent (LAD) artery which involved intervening via the left internal mammary artery (LIMA) graft. The other three cases all involved lesions at or beyond the anastomoses of a SVG to the native coronary vessels. These cases were chosen on the basis of their complex anatomies and were graded according to a complexity score that took into account both the vessel and lesion characteristics12. The methodology used was in accordance with a previously published study whereby all operators were trained in both conventional and magnetically guided PCI, and were additionally allowed unrestricted access to an assortment of conventional and magnetic guidewires. A 3-D roadmap created from two orthogonal radiographic images was used to facilitate the magnetic navigation of the wire through the vessel.
Although the study was not powered to provide any statistical inference, by virtue of the MNS ability to redirect the wire in situ, an almost three-fold decrease in crossing times was observed in the cases involving LIMAs (1041.3 sec vs. 391.7 sec) and a near two-fold decrease in the SVGs (1037.3 sec vs. 680.3 sec). Fluoroscopic times were similarly reduced in both LIMA (970.7 sec vs. 343.3 sec) and SVG cases (908 sec vs. 584 sec). Moreover, as a direct consequence of the visual roadmap, there was a dramatic reduction in contrast media usage with the MNS (6 mls vs. 35.3 mls in LIMA and 15.3 mls vs. 27.7 mls in SVG cases) compared to the standard wire techniques.
Selective failed conventional wire CABG cases managed successfully with the MNS
During the same study period (Oct 2006 - Sept 2007) there were three failed conventional wires cases that were subsequently crossed using the MNS. Two of these cases are described below in the cases studied in order to illustrate the various techniques and navigational modes that can be used to achieve procedural success.
CASE 1
A 68 year-old hypertensive patient presented with class II anginal symptoms. Eight years previously he underwent CABG with a LIMA to the LAD together with SVG to the posterior descending artery (PDA) and the 1st obtuse maginalis artery. Elective angiography revealed a severe stenosis at the bifurcation of the 1st diagonal branch, which on cardiac MRI demonstrated reversible ischaemia. The native left main stem (LMS) was calcific and severely stenosed with severe disease extending into the proximal LAD. An antegrade approach to revascularisation with conventional techniques was attempted but failed as the wire could not be successfully tracked beyond the calcific distal LMS. For the same procedure, a retrograde approach from the LIMA to the proximal LAD into the diagonal branch was attempted. (Figure 2) Despite using several conventional wires (3 x Pilot 50, 2 x Balanced Middle Weighted and 2 x Whisper wires), all having different manually created angulated bends at the tip, the wires were unable to engage beyond the ostium of D1. After a prolonged procedure (approximately 2 hours) using almost 500 mls of contrast media the procedure was abandoned and a magnetic assisted intervention was planned for the patient.
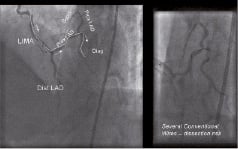
Figure 2. Retrograde approach from LIMA to the proximal LAD and diagonal branch attempted with conventional wires. The lack of control of the wire tip associated with multiple bends, increased the risk of vessel dissection. See text for details.
The magnetic navigation was performed using a virtually created roadmap derived from two orthogonal radiographic images of the distal LIMA and the native coronary arteries (Figure 3). By following co-registration to the real time fluoroscopic images, the associated navigation centreline and vectors were used to change the orientation of the wire tip along the vessel in accordance with a chosen path. Using the navigational path for retrogradely entering the D1, a 2 mm Titan angulated tip magnetic wire was able to traverse D1 distally using only 10 ml of contrast media in a relatively short crossing time (2 minutes) from the ostium of the LIMA (Figure 3).
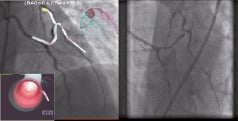
Figure 3. Virtual roadmap derived from two orthogonal radiographic images of distal LIMA and native coronary arteries. An endoluminal reconstruction (inserted) was used during magnetic navigation to guide a 2 mm magnet Titan angulated guidewire into the 1st diagonal artery.
The wire also supported an over-the-wire (OTW) balloon that was used to predilate the severe ostial D1 lesion. After serial balloon dilatation, a drug eluting stent was successfully deployed in D1 and extended proximally into the distal LAD. Following post-dilatation of the stent, pinching of the proximal LAD was apparent, but was successfully managed by recrossing the overlying struts using a similar magnetically enabled wire together with vectors associated with the navigational path derived using the proximal LAD. Subsequent opening of the struts with a small 2 mm diameter balloon resulted in a satisfactory angiographic result (Figure 4).
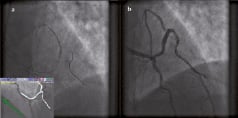
Figure 4. Panel A: Following post-dilatation of the stent, pinching of the proximal LAD became evident. This was successful managed by recrossing the overlying struts with a similar magnetically enabled wire, using MNS and a navigational path extended to the proximal LAD. Panel B: Final angiographic result.
CASE 2
This is the case of a 70 year-old male patient with a complex coronary history and progressively worsening angina on optimal medical therapy. His risk factors included hyperlipidaemia and hypertension, also he had undergone a percutaneous coronary intervention (PCI) to the right coronary artery (RCA) in 2002 following an acute myocardial infarction. Seven years later, as a consequence of multivessel disease and these symptoms he underwent a coronary artery bypass grafting (CABG) with a LIMA to LAD and SVG to ramus intermediate and a jump graft to the 1st Diagonal. Recurrent anginal symptoms six weeks following this surgery prompted coronary angiography that revealed a sub-total occlusion at the junction of the SVG graft to the intermediate ramus. As a result of the acute angulation at the anastomosis to the native vessel, PCI using conventional methods and guidewires was unsuccessful. Although adequate support was achieved with a Judkins Right 4 (JR4) guide catheter by “deep throating” it into the graft, the initial bend made on the standard guidewire could not adapt to the near right angle needed to cross the lesion (Figure 5). Several wires of varying stiffness (2 x Pilot 50, 2 x Balanced Middle Weighted, 1 x Whisper and 1 x Miracle 3 gram wires) all failed, despite some being supported by an over-the-wire balloon (OTW). In order to facilitate navigation in this complex anatomy the patient had a planned Stereotaxis magnetic navigation system (MNS) procedure the next day.
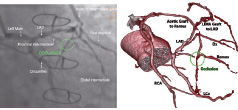
Figure 5. Severe stenosis at the anastomosis of the SVG to the ramus intermediate artery that proved unsuccessful to cross because of the hairpin turn followed by a right angle bend, here best seen on the CTCA on the right panel.
A co-integration of the CTCA and the MNS was thought to be a good strategy for guiding through the complex anatomy at the anastomosis. This was due to the fact that the sub-totally occluded vessel does not lend itself to a 3-D reconstruction because of lack of contrast media in the occluded segment. The use of CTCA, however, can circumvent this limitation and, moreover, can display the multi-planer reconstructive segment to “fine-tune” guidewire navigation (Figure 6). But before the operator can utilise the virtual navigational roadmap to automatically traverse down the vessel using targeted magnetic fields, the CTCA must first be co-registered with the live fluoroscopic images. This is performed by adjusting the ostium of the SVG to the position of the guide catheter in two orthogonal fluoroscopic planes as shown in Figure 6.
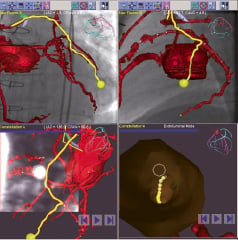
Figure 6. Co-registration of the preprocedural CTCA to the fluoroscopic images in two orthogonal planes showing the pathway depicted as a yellow line through the vessel. In the bottom panel, the multi-planer reconstructive (MPR) slice through the vessel is updated together with the vector and the endoluminal view can be used to further refine the direction of the vectors.
Once this was achieved, it was then possible to prospectively trace the path of the vector though the vessel and, moreover, look through the vessel lumen via the virtually created endoluminal view (Figure 6). The Stereotaxis Pegasus Moderate Support 2 mm angled guidewire was preferentially chosen for this challenging hairpin turn followed by another 90° turn, as this 3rd generation wire offered the highest degree of angulation (131-137°) to the applied magnetic field. By employing the vectors as illustrated in Figure 7, the Pegasus wire was able to successfully cross into the native vessel and had sufficient pushability to engage both turns. It also enabled an over-the-wire balloon to be successfully tracked in order to facilitate stenting.
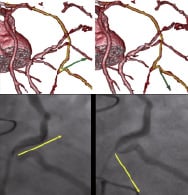
Figure 7. The Pegasus Moderate Support 2 mm angled guidewire was able to successfully traverse through this complex anatomy by using the vectors derived from the CTCA.
The procedure was concluded by the complete revascularisation of the native vessels by antegradely refashioning the LAD and the intermediate by deploying a Tryton Side-Branch protecting stent into the intermediate/LMS and then stenting into the LMS-LAD with a drug eluting stent followed by a kissing balloon dilatation (Figure 8).
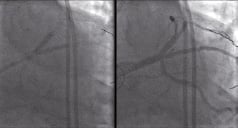
Figure 8. Antegradely refashioning the LAD and the intermediate with a Tryton Side-Branch stent into the intermediate/LMS, then stenting into the LMS-LAD with a Xience V drug eluting stent followed by a kissing balloon dilatation to give the final result on the right panel.
Discussion
Secondary revascularisation after CABG remains a challenge for both surgeons and interventional cardiologists. The associated higher surgical mortality and morbidity as well as limited conduit availability may be decisive in certain patient subsets in choosing PCI approach. In the BMS era, PCI of SVGs was associated with worse outcomes and a high incidence of in-stent restenosis13. Even though there is limited randomised data, the use of DES is encouraging, despite the perception that higher local prothrombotic conditions in SVG, together with the delay in endothelial healing after DES, may lead to higher rates of acute, subacute, and late stent thromboses. In addition, the friable and eccentric nature of the plaque disease in SVG has the potential risk of increasing complications, particularly related to vascular dissection. Crossing these lesions with a technology that can limit vascular trauma is therefore highly desirable, especially if it offers the ability to negotiate through the various acute angulations typically encountered at the anastomotic site. Moreover, patients with prior CABG present a higher prevalence of left ventricular dysfunction and chronic renal impairment thus having a technology that can lower contrast media usage is perhaps particularly applicable in this population.
Magnetic navigation is still a relatively new technology in the field of interventional cardiology. Over the past five years there has been major advances, both in the software and hardware14. The system has demonstrated advantages by reducing both crossing and fluoroscopy times as well as contrast media usage, particularly as lesion complexity increases. As far as its extension to secondary revascularisation is concerned, there is limited data available to draw definitive conclusions. Certainly, in the small, randomised patient population, the most obvious gain was in contrast agent reduction and this may be directly related to the visually aided roadmap the system displays on the live fluoroscopic image. There was also a marked reduction in crossing and fluoroscopy time, but a much larger study is warranted to further evaluate this fully. Far more significant, however, was the ability of the system to salvage failed conventional cases, especially after prolonged procedural times, contrast media usage and an armamentarium of several different conventional wires failing to enter difficult angulations. The MNS reduced the crossing times in these extreme cases from hours to a few minutes, emphasising the importance of having the “right tool for the right job” as well as removing the “trial and error” approach of making the “right bend on the wire” that will support the wire to it’s final destination.
There are several limitations, however, with the MNS, that has thus far limited its more widespread acceptance within the interventional community. The high cost related to the hardware and the associated technical demands make it difficult to currently justify the rather limited advantages in a select patient population. Also, some of the limitations of current magnetic navigational wires needs to be addressed. In particular, the size of the magnet (2-3 mm) constitutes, at this stage of development of the technique, a limitation in navigating acute bends. But this is a rapidly evolving field, and as demonstrated by the second case study in this paper, the newer generation wires have already improved crossing profiles. The multi-magnet design guidewire in development today has two or three shorter magnets at the tip which may offer advantages in navigating acute bends over the current single (2-3 mm) magnetic tip.
The ability of the system to integrate 3-D dataset to perform a PCI is definitively a glimpse into the future, where it is predicted that noninvasive CTCA may supersede the use of angiography for both screening and lesion characteristics15. The use of CTCA in the evaluation of grafts can avoid the limitations of vessel overlap and the poor lesion/anastomotic evaluation associated with conventional luminology. In addition, CTCA and co-registration of the internal mammary arteries dataset, can facilitate effective intervention through the supra-aortic vessels in order to save both time and contrast media usage. Moreover, as demonstrated in this report, the marriage of a 3-D dataset with the MNS can turn a failed conventional wire technique case into a procedural success.
Conclusion
The Magnetic Navigation System (MNS) has been successfully used in the secondary revascularisation of failed conventional CABG cases. Retrograde PCI through a LIMA is not only feasible, but the wires can manage stenoses involving a bifurcation using 3D reconstruction software. Difficult anatomies, such as the hairpin bend typified at a SVG anastomosis, can be overcome by co-integrating a CTCA 3D dataset for navigation. Preliminary data supports potential advantages in reduction of contrast media usage, crossing and fluoroscopy times and suggests that larger randomised studies are warranted.
Acknowledgements
We thank Heather Drury and Diran Guiliguian of Stereotaxis for contributing to the Figures 5 and 7.

