Abstract
Aims: To investigate the safety and feasibility of a newly developed magnetic navigation system for intracoronary tracking.
Methods and results: The MediGuide Medical Positioning System (MPS) is a navigation system that was developed to facilitate the navigation of enabled devices within the coronary tree using a magnetic tracking technology. The current prospective, non-randomised, single-centre, first-in-man study was conducted at Universitätsklinikum Regensburg (UKR), Germany on an MPS-enabled AXIOM Artis dFC coronary angiography system (Siemens AG, Forchheim, Germany). We enrolled 20 patients who required IVUS assessment or treatment of a single de novo target lesion in a native coronary artery. The performance was evaluated on a semi-quantitative one-to-five scale where a score of five indicates an excellent superimposition with the vessel and a score of one an unacceptable performance. The mean score for tracking as assessed by projection on life fluoroscopy was 4.89 and 3.58 as assessed by projection on recorded cine-loop. Length measurement of a 20 mm distance was significantly better with the MPS (mean deviation of 0.6 mm=3%) as compared to standard QCA (1.5 mm=8%, p<0.05). Creating a 3D reconstruction was possible in 13 out of 20 cases with an average score of 4.68. No adverse events occurred.
Conclusions: The MediGuide Medical Positioning System is safe and feasible in man, facilitates intracoronary navigation and allows 3D reconstruction of the investigated coronary segment.
Introduction
Coronary angiography and intervention require significant amounts of contrast dye and radiation exposure. Although much progress has been made with respect to x-ray technology in recent years, a further reduction in radiation and contrast dye exposure is highly desired by both operators and patients. Recent efforts like the introduction of the magnetic steering system from Stereotaxis (Stereotaxis, St Louis, MO, USA) to manipulate electrophysiology catheters or guidewires in coronary procedures may overcome many limitations of conventional angiographic procedures1,2, but this system requires special room preparation and much higher investment.
As an adjunct to the x-ray, a new magnetic based navigation modality has been developed. In addition to tracking a device (e.g. PTCA balloon) on the x-ray image by observing the radiopaque marker, the position and orientation of an enabled device are measured by means of a magnetic system in the three-dimensional (3D) space which then allows device location relative to the 2D x-ray image. As the reliability and quality of electromagnetic tracking systems increases, electromagnetic navigation has more potential for providing guidance in invasive procedures. Such magnetic systems are already in commercial use in electrophysiological applications1-4, bronchoscopic biopsy5, and for tracking of various medical devices in the fields of image guided surgery6. These systems are usually installed in addition to existing x-ray systems.
The MediGuide Medical Positioning System (MPS system) was developed to enable tracking and navigation of specific devices within the coronary tree. It enables real time positioning and navigation of any device equipped with an MPS sensor. Such a device, bearing a CE mark, is the Guided Measurement Catheter (GMC™). The system is used as an adjunct to conventional coronary angiography and, if proven accurate, might ultimately improve the quality of coronary imaging and possibly also interventions despite reduced radiation and dye exposure7,8.
The current first-in-man study was a prospective, non-randomised, feasibility study that enrolled 20 patients for assessment of the MPS system in conjunction with the GMC device. Patients who required IVUS assessment or treatment of a single de novo target lesion in a native coronary artery and who met eligibility criteria were enrolled into the study after a suitable stenosis was diagnosed by means of conventional angiography.
Methods and material
Subjects
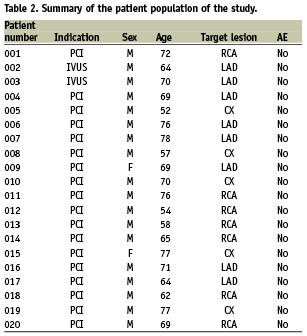
The main inclusion criteria for enrolment in the study were age older than 18 years, stenosis higher than 50% in a native coronary segment, no atrial fibrillation or extensive premature beats and stable angina. Overall, 44 patients signed the informed consent form. From this group, 20 patients (18 men, two women, mean age 67,5±7,8 years) were finally enrolled in the study. The 24 patients who were excluded were found unsuitable after the diagnostic angiogram was performed because of violation of inclusion or exclusion criteria. Most often, no coronary stenosis requiring PCI was present, other reasons were extensive premature supraventricular or ventricular contractions or lesions narrower than the GMC crossing profile. All patients were followed until discharge from our institution including laboratory analyses, ECG and physical examination The study was approved by the local ethical committee of Universitätsklinikum Regensburg, Germany (EC Nr. 07/076).
Technical description
In this clinical investigation, we investigated the Medical Positioning System (MPS) in conjunction with the Guided Measurement Catheter (GMC™), a technology which was developed by MediGuide Ltd., Haifa, Israel. The set-up has already been described in detail in a previous technical report8. Briefly, hardware and software elements were installed on a Siemens AXIOM Artis dFC (Siemens AG, Forchheim, Germany) (Figure 1). The conventional x-ray imaging system equipped with the MPS elements performs as intended for angiographic imaging, while enabling catheter tracking and enhanced visualisation tools supplied by the MPS capabilities. A magnetic reference sensor (similar to an ECG-electrode in size) is attached to the patient’s chest to provide information about the spatial relationship between the chest wall and the flat image detector, and is used to compensate for respiratory movements. The catheter used with the MPS system is the Guided Measurement Catheter (GMC). The GMC rapid exchange device is a balloon-like coronary catheter, equipped with a miniaturised localisation sensor at its tip. It has a crossing profile of 1.15 mm being compatible with a 6 Fr coronary guiding catheter and a 0.014» PTCA guidewire. The sub-millimetre MPS sensor senses the low intensity magnetic field (less than 200 µTesla nominal value) generated by the MPS and generates an electrical signal out of which the accurate real-time position and orientation information is extracted. Thus, the catheter is tracked continuously while in the confined volume controlled by the low magnetic system in and around the patient’s heart. The co-registration of the MPS with the imaging system provides for projection of the tip’s position and orientation onto live fluoroscopy and recorded cine-angiography as well as onto reconstructed 3D images of the investigated coronary segment. The GMC catheter is continuously tracked while it is pushed forward and pulled back. (Figure 1)
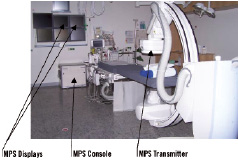
Figure 1. The MPS components installed in the catheterisation suite of UK Regensburg in conjunction with the Axiom Artis dFC.
As the pullback is completed, the 3D trace of the catheter’s trajectory is reconstructed and displayed. The trace is colour coded to indicate the angular percent of foreshortening of the artery as seen in the respective x-ray image (Figure 2, left panel). A model of the artery’s lumen is automatically generated from the positioning information collected by the catheter sensor and segmentation of the artery. The contour is given by the segmentation of one angiographic image, selected by the user from the reference cine-loop. The image is selected so as to show the lumen of the investigated artery enhanced by contrast agent. The spatial shape of the 3D model is given by the 3D trace, collected during the catheter’s manual pullback.
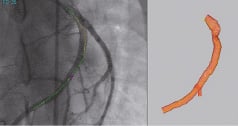
Figure 2. Smart Trace superimposed on a cine-loop (left) and the 3D model (right).
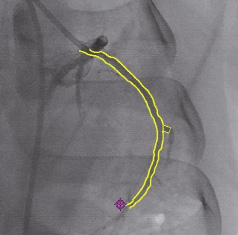
Figure 3. The luminal model’s contour superimposed on an X-ray image.
Thus, the model can be visualised as a 3D independent object and also superimposed on and registered with the X-ray image (Figure 3). An additional feature of the system allows the user to landmark a point of interest (POI), superimposed on the 2D X-ray (real-time and cine-loop) and on the 3D model. This 3D mark can be used to point to an anatomical landmark and is displayed on any image and from any viewing angle (Figure 4).
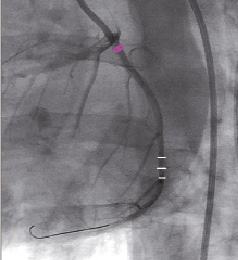
Figure 4. Virtual landmark on the 2D Display, situated proximally to a side-branch.
Length measurements can be performed on the 3D trace, in the 3D display or on the smart trace projected on the X-ray image to measure any segment along the trajectory followed by the catheter. Measurements of a lesion can be performed assessing both length and cross-sectional diameter and area.
Procedure flow
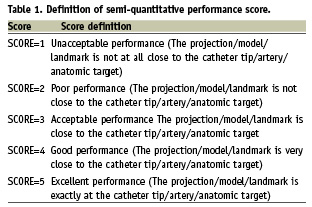
After a suitable lesion was detected by conventional angiography, a graded guidewire (IQ™, Boston Scientific, Natick, MA, USA) was advanced beyond the lesion to the distal part of the coronary artery after administration of 70IE heparin/kg body weight. Then, a reference cine-loop was recorded with contrast agent injection. The IQ guidewire was delivered as per standard procedure. The GMC device was advanced over the wire to the distal end of the guiding catheter and forward until the catheter tip projection marker crossed a minimum of 1 cm (0.39 inches) beyond the region of interest in the target vessel segment. During insertion and pullback, the MPS position was shown in real-time on both, live fluoroscopy and recorded cine-loops, the later on the additional monitor part of the MPS system. After pullback of the GMC catheter, a smart trace was automatically created and displayed on the 3D display as well as superimposed on the 2D x-ray image (Figure 2). To generate the 3D model, a single angiographic frame (from the reference cine-loop) was selected by the investigator and automatic segmentation of the arterial lumen followed by 3D model reconstruction was performed and presented (Figure 2). Then, 2D and 3D QCA measurements were performed. Lastly, the investigator marked the virtual landmark on a lesion or at a bifurcation area. The accuracy of the projection of the MPS position on live fluoroscopy and on recorded cine-loop as well as the accuracy of the 3D-reconstruction and the virtual landmark were scored on a semi-quantitative one-to-five scale, ranging from poor (one) to excellent (five). The score was defined as depicted in Table 1.
At last, length measurements of the graded guidewire using conventional QCA to be compared to the MPS results were performed. The graded guidewire, having well defined radiopaque markers, served as gold standard for the two measurement methods and was used to assess the accuracy of the MPS length measurements relative to the conventional QCA tools used by the angiographic system.
Statistics
For all statistical calculations the SPSS software 13.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used. For parametric variables, the Students t-test was applied, whereas the X2 test was used for non-parametric variables. All numbers are given as mean ±SD (standard deviation). Differences were considered statistically significant at a p<0.05.
Results
Patient characteristics
The patient characteristics are depicted in Table 2. In two cases, an IVUS study was performed after the MPS procedure whereas a coronary intervention was performed after the diagnostic angiography and MPS evaluation in the other 18 patients. No adverse events were recorded neither during intra-coronary investigation with the GMC device, neither during the subsequent cardiac intervention (PCI/IVUS), nor during follow-up until discharge as assessed by laboratory analyses, ECG and physical examination.
Intracoronary tracking and navigation
In 19 out of the enrolled 20 patients, the projection of the GMC on live fluoroscopy was graded by two investigators. In one out of twenty patients, the MPS assessment was not performed and the GMC device was removed because of a too tight target lesion. The mean score was 4.89±0.31 (twice grade 4, 17 times grade 5), indicating an excellent accuracy of intracoronary magnetic tracking. The mean score of the MPS projection on a formerly recorded cine-loop was lower with an average score of 3.57±1.17. A smart trace was generated and displayed in all 19 performed cases.
Three-dimensional luminal model and virtual landmark
The 3D model was successfully reconstructed in 16 out of 20 cases and the accuracy of the reconstructed 3D model of the vessel segment resulted in a score of 4.68±0.48. In four cases, the 3D model could not be created because of insufficient motion compensation due to irregular breathing patterns. The last scoring during the procedure was the accuracy of the virtual landmark, which was set on a bifurcation, a side-branch or a stenosis using the 3D model and then compared to the projection on the live fluoroscopy. The average score was 3.50±0.65. Figure 5 summarises the mean scores of the four different performance parameters.
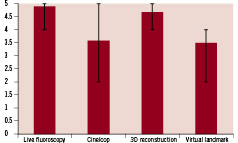
Figure 5. Mean scores of all performed cases for performance of the MPS parameters. Bars extend to minimum and maximum reached in individual studies.
MPS length measurements versus quantitative coronary angiography
Following the procedure, conventional QCA length measurements were done using the marker of the IQ guidewire as gold standard to be compared to the MPS measurements of the same distance (Figure 6). The mean difference of the QCA length measurements compared to the guidewire spacing was 1.5±1.2 mm or 8±6 percent. The deviation of the MPS length measurements was significantly lower with 0.6±0.6 mm or 3±3 percent (p<0.05).
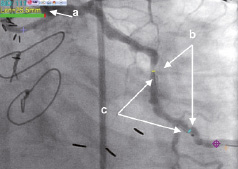
Figure 6. Example of 3D measurements on a 2D image. a. Measurement result (25.5 mm), b. Measured segment delineated by 3D tool’s markers, c. Marker guidewire radiopaque markers.
Discussion
In the current first-in man study, the magnetic positioning system, operated in conjunction with the MPS enabled GMC device, has demonstrated that intracoronary navigation through magnetic tracking in man is feasible with extraordinary precision. The current experience suggests that intracoronary magnetic navigation with enabled devices is an emerging technology with a great potential to improve coronary imaging and precise navigation.
Intracoronary navigation
A navigation technology which aims to improve the quality of percutaneous coronary interventions with the potential future capability to reduce radiation and dye exposure must demonstrate above all excellent spatial tracking. In this first-in-man study, the MPS system in conjunction with a GMC catheter has clearly met this goal. Particularly the high scoring values regarding the accuracy of the projection of the MPS-enabled GMC catheter onto the vessel lumen during live fluoroscopy demonstrates the highly accurate tracking of real-time position and trace of the magnetic sensor despite continuous movement of the coronary artery.
Due to the highly accurate tracking capabilities of the MPS system, potential future applications may include implementation on IVUS which would then allow for a true 3D vessel and vessel wall reconstruction, on guidewires and on PCI-balloons and/or stents which would then allow for highly accurate navigation and positioning. For future trackable devices, most of the benefit will be achieved from the actual tracking and not only after crossing a lesion.
In contrast to these excellent results, lower grades were observed for the projection on recorded cine-loops which allow intracoronary navigation without concurrent fluoroscopy. The underlying reason is not the accuracy of the spatial tracking of the MPS sensor but rather the challenge to superimpose the real time positioning information (MPS) with the stored, pre-recorded (cine-loop) signal. This superimposition requires a compensation for temporal movements resulting from irregular breathing, chest or patient movements. If such compensation is not highly accurate, the real-time (MPS) signal will not superimpose with the pre-recorded (cine-loop) signal and falsely give an impression of inaccurate tracking.
In the current study, the compensation was performed through co-registration of a reference sensor which was attached to the cranial part of the patients sternum and allowed to continuously register and compensate for breathing excursions and movements. Despite this compensation, however, differences between the actual position of the MPS probe and the pre-recorded cine-loops were observed, particularly in patients with very little chest movements during respiration due to chronic obstructive lung disease or obesity and resulted in lower overall grades for the projection on recorded cine-loops and accuracy of virtual landmarks. Therefore, the superimposition of real time (MPS) and stored, pre-recorded (cine-loop) signal requires further refinement, being more a computational challenge than a problem of the magnetic technology per se. The motion compensation algorithm is currently undergoing further improvement and will be included in the next generation of the system.
Three-dimensional luminal model and virtual landmark
In addition to the real-time position of the magnetic sensor, the MPS system provides a function which allows visualising the trace of the sensor located at the catheter tip. The trace is recorded during a pullback- or push-in manoeuvre through the coronary artery and presented after post-processing as three-dimensional reconstruction with colour-coded indication of vessel foreshortening. Additionally, a three dimensional model of the inspected coronary segment can be generated from the MPS trace and the vessel luminal contour obtained from the angiographic image segmentation. Both features, trace and three dimensional model of the inspected coronary segment also allow for length measurements and lesion size determination.
In the current study, precise length measurements (evaluated relative to graded guidewire) were possible by use of this trace and were significantly more accurate as compared to conventional x-ray quantitative coronary analysis (QCA) which, as a 2D method, is highly susceptible to foreshortening and angulation artifacts. The high spatial accuracy of the magnetic positioning system is due to the sub-millimetre tracking of the MPS probe and the information which becomes available through the trajectory and path of the MPS probe and is particularly advantageous in cases of severe foreshortening and angulated vessel segments. The accuracy of the trace is
further indicated by the high scores which were achieved for the reconstructed three dimensional luminal model of the inspected coronary segment by visual comparison with the angiographic anatomy.
Since the MPS performance is much influenced by the patient’s spontaneous cardiac and respiratory motion, as well as by body movements, the virtual landmark, which is particularly sensitive to body movements, showed relative lower performance. In future versions of the application, this parameter’s performance will improve as well by further improvement of the motion compensation algorithm.
Limitations
Since the magnetic positioning system in its current form is purely diagnostic, current limitations include the necessity to cross the lesion with the device including a risk of vessel trauma. Further current limitations are slightly restricted angulation and radiation scatter due to the increased size of the prototype cover. This drawback will be eliminated in the future by reducing the size of the transmitters and restoring the original functionality of the anti-scattering grid.
Conclusions
The MPS is an emerging, feasible technology which allows for tracking of real-time position and trajectory of the MPS-enabled device. The system has demonstrated an excellent safety profile with no adverse events. It facilitates intracoronary navigation and allows 3D reconstruction of the investigated coronary segment.
1. Kiemeneij F, Patterson MS, Amoroso G, Laarman G, Slagboom T. Use of the Stereotaxis Niobe magnetic navigation system for percutaneous coronary intervention: results from 350 consecutive patients. Catheter Cardiovasc Interv. 2008 Mar 1;71:510-6.
2. Ernst S, Ouyang F, Linder C, Hertting K, Stahl F, Chun J, Hachiya H, Bänsch D, Antz M, Kuck KH. Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation. 2004; 109:1472-5.
3. Ben-Haim SA, Osadchy D, Schuster I, Gepstein L, Hayam G, Josephson ME. Nonfluoroscopic in-vivo navigation and mapping technology. Nat Med 1996; 2:1393-5.
4. De Groot NM, Bootsma M, van der Velde ET, Schalij MJ. Three-dimensional catheter positioning during radiofrequency ablation in patients: First application of a real-time position management system. J Cardiovasc Electrophysiol 2000;11:1183-1192.
5. Becker HD, Herth F, Ernst A, Schwarz Y. Bronchoscopic biopsy of peripheral lung lesions under electromagnetic guidance: a pilot study. Bronchology 2005;12: 9-13.
6. Tang, Cleary K. Breakdown of tracking accuracy for electromagnetically guided abdominal interventions. Proc CARS 2003,452-9
7. Strommer G, Schwartz L, Shmarak I, Flugelman MY, Shiran A, Sobe L, Oren E, Shofti R, Leon MB, Lewis BS. Fluoroscopy free navigation of Guided-Stent using Medical Positioning System and 3D Guided-IVUS (GIVUSTM) image. International Congress Series 2005;1281:387-392.
8. Flugelman M, Nusimovici-Avadis D, Schwartz DL, Herscovici A, Cohen A, Luchner A, Jeron A. Medical Positioning System: Technical Report. EuroIntervention 2008;4:158-160.

