
Abstract
Aims: The aim of this study was to analyse the real-world national data on parallel utilisation of transcatheter (TAVR) and surgical (SAVR) aortic valve replacement.
Methods and results: We queried an all-payer, administrative United States in-patient database to identify all AVR hospitalisations in patients aged ≥18 years from January 2012 to December 2016 and examined the temporal changes in the number of AVR procedures and in-hospital mortality. A total of 463,675 AVRs were performed – 363,275 (78.4%) SAVR and 100,400 (21.6%) TAVR. AVR linearly increased (from 78,985 in 2012 to 103,415 in 2016; +30.9%; ptrend<0.001) largely due to a marked increase in TAVR (from 7,655 to 33,545; +338%; ptrend<0.001), whereas the absolute number of SAVRs remained relatively stable (from 71,330 to 69,870; –1%; ptrend<0.001). The number of TAVRs increased in all pre-specified age groups (<75, 75-79, 80-85, and ≥85 years; ptrend<0.001 for all). In contrast, the number of SAVRs increased modestly in patients aged <75 years (ptrend<0.001) and declined in those aged 75-79 years, 80-84 years, or ≥85 years (ptrend<0.001 for all). Age- and sex-adjusted in-hospital mortality after isolated (aOR 1.00 [0.95-1.05]; ptrend=0.96) or combined SAVR (aOR 1.01 [0.97-1.05]; ptrend=0.66) remained unchanged during the study period, whereas in-hospital mortality after TAVR declined (aOR 0.75 [0.70-0.79]; ptrend<0.001). Similar trends in in-hospital mortality were seen in the age subgroups.
Conclusions: The number of AVRs markedly increased in the USA from 2012 to 2016, mainly due to the widespread adoption of TAVR, whereas the number of SAVRs remained relatively stable. In-hospital mortality after TAVR declined, whereas that after SAVR has remained unchanged.
Introduction
Aortic stenosis (AS) is the second most common valvular heart disease in Western countries and its prevalence increases with age1. Severe, symptomatic AS is associated with an adverse prognosis if left untreated; the benefits and indications of aortic valve replacement (AVR) are well defined in clinical practice guidelines2. However, approximately one third of the patients with an indication for AVR are denied surgical AVR (SAVR) due to an increased perioperative risk related to advanced age, comorbidities, left ventricular dysfunction, anatomical, and other factors3. In the last decade, transcatheter aortic valve replacement (TAVR) has emerged as an alternative to SAVR in patients considered at prohibitive, high, or intermediate risk for perioperative mortality after SAVR4. Recent data have established the efficacy and safety of TAVR even in patients at low surgical risk. The indication for TAVR is expected to be extended to this patient subset in the near future5,6. Data from Germany suggest that the number of TAVRs has caught up with the number of SAVRs4,7. It is important to examine the impact of adoption of TAVR on the number of SAVRs and overall number of AVRs in the USA. In this study, we aimed to: 1) assess the number of AVRs performed in the USA and changes over time; 2) assess the adoption of TAVR and SAVR in age-stratified subgroups; and 3) examine the temporal trends in in-hospital mortality after AVR by procedure type.
Methods
DATA SOURCE
Data were obtained from the National Inpatient Sample (NIS) database files from January 2012 to December 2016. The NIS is a database of hospital inpatient stays derived from billing data submitted by hospitals to statewide data organisations throughout the USA. These inpatient data include clinical and resource use information typically available from discharge records. The NIS is an all-payer database and currently includes data from 47 states of the USA, covering >97% of the population of the USA. The NIS approximates a 20% stratified sample of discharges from participating hospitals. Discharge weights provided for each record can be used to generate national estimates8.
STUDY POPULATION
We used the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) or International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM) procedure codes to identify all patients aged ≥18 years undergoing TAVR or SAVR. The hospital administrative system in the USA transitioned to use of ICD-10-CM codes beginning from October 2015; therefore, data from October 2015 onwards were extracted using ICD-10-CM codes. We further categorised the SAVR group to those undergoing isolated SAVR or combined SAVR (defined as concomitant coronary artery bypass grafting [CABG]) or other valvular surgery. The ICD-9-CM or ICD-10-CM procedure codes used to identify the study population are provided in Table 1.
BASELINE CHARACTERISTICS AND OUTCOMES
Baseline characteristics included were age and sex. Our study outcome of interest was in-hospital mortality.
STATISTICAL ANALYSIS
Survey-specific techniques accounting for the multi-level nature of the data were used for weighting to obtain national estimates, as recommended by the Agency for Healthcare Research and Quality9. Poisson regression analyses were used to examine the changes in the number of AVRs over time in the overall study population and in pre-specified age subgroups (<75 years, 75-79 years, 80-84 years, and ≥85 years). Complex samples multivariable logistic regression analysis was used to study the temporal trends in age- and sex-adjusted in-hospital mortality. Statistical analysis was conducted using SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA). All p-values are two-sided with a significance threshold of <0.05. Odds ratios (OR) and 95% confidence intervals (CI) were used to report the results of regression analyses.
Results
BASELINE CHARACTERISTICS
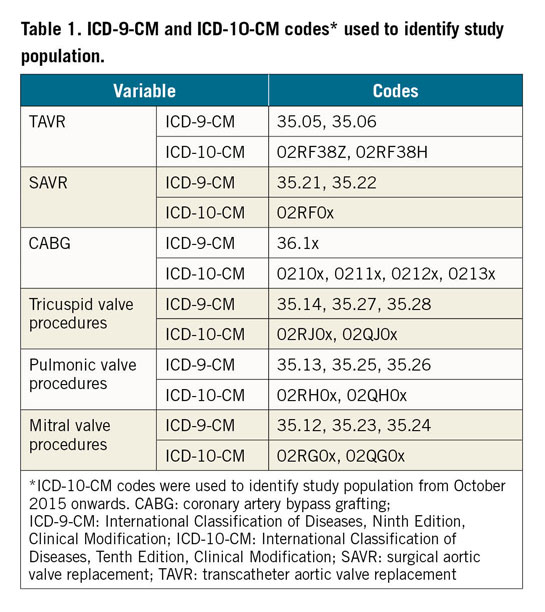
Between January 2012 and December 2016, there was a total of 463,675 AVRs performed in the USA: 363,275 (78.4%) were SAVRs and 100,400 (21.6%) were TAVRs. SAVR was performed in isolation in 192,475 patients (53.0% of all SAVRs), whereas combined CABG was performed in 81,155 (22.3%) and a concomitant valvular surgery in 48,025 (13.2%). Concomitant CABG and other valvular surgery were performed in combination with SAVR in 41,620 patients (11.5% of all SAVRs). There was a slight decrease in the mean age of patients undergoing either TAVR (from 81.1 to 80.4 years; ptrend<0.001) or isolated SAVR (from 67.9 to 64.3 years; ptrend<0.001) during the study period, whereas the mean age of patients undergoing combined SAVR increased slightly (from 69.2 to 69.6 years; ptrend<0.001) (Table 2).
NUMBER AND TYPES OF AVRs
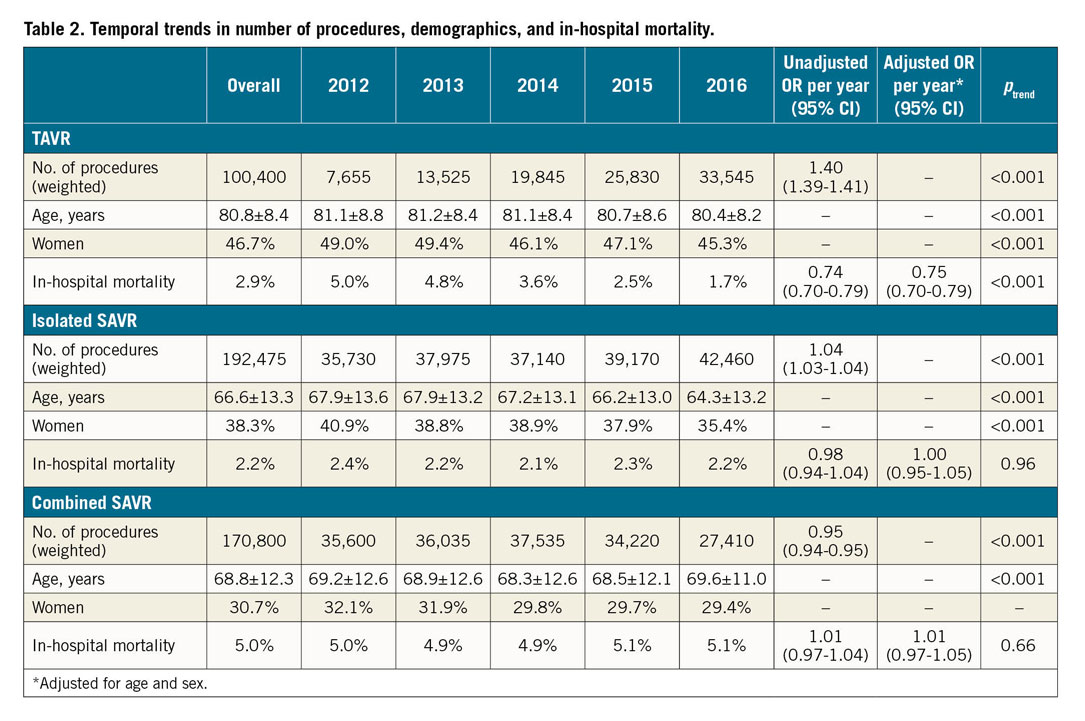
The total number of AVRs increased linearly during the study period (from 78,985 in 2012 to 103,415 in 2016; OR 1.07 [1.06-1.07]; +30.9%; ptrend<0.001). This was driven mainly by a marked increase in the number of TAVRs (from 7,655 to 33,545; +338%; OR 1.40 [1.39-1.40]; ptrend<0.001), whereas the number of SAVRs remained relatively unchanged (from 71,330 to 69,870; -1%; OR 0.997 [0.993-0.997]; ptrend<0.001) (Table 2, Figure 1). Of all AVRs, the proportion of TAVR increased from 9.7% in 2012 to 32.4% in 2016 with an accompanying decrease in the proportion of isolated (from 45.2% to 41.1%) or combined SAVR (45.1% to 26.5%) (Figure 2).
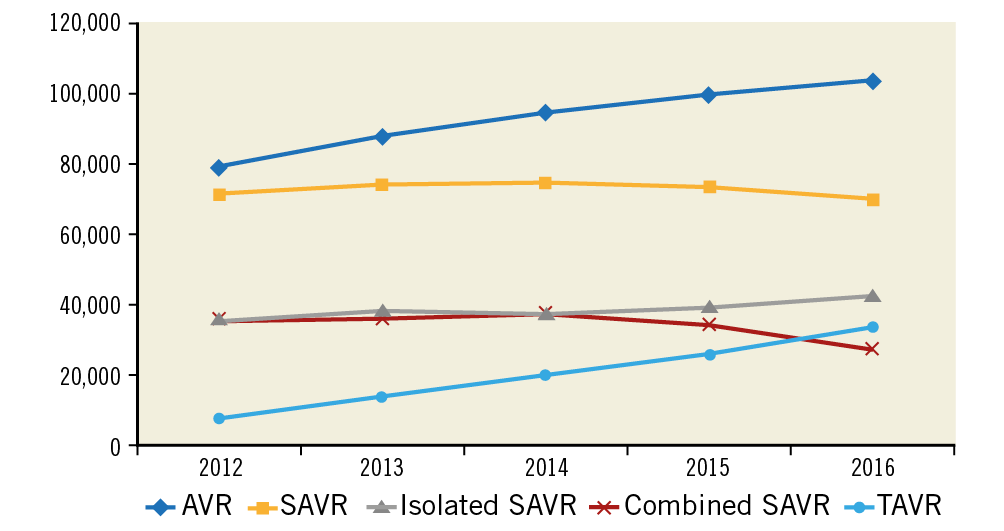
Figure 1. Trends in number of AVRs performed by type from 2012 to 2016. ptrend<0.001 for all.
Figure 2. Trends in the proportion of surgical aortic valve replacement (SAVR) either isolated or combined and transcatheter aortic valve replacement (TAVR) between 2012 and 2016.
The number of TAVRs increased in all age categories (<75, 75-79, 80-85, and ≥85 years; ptrend<0.001 for all). In contrast, the number of SAVRs increased modestly in patients aged <75 years (+11.3%; ptrend<0.001) and declined in those aged 75-79 years, 80-84 years, or ≥85 years (–7.2%, –34.5%, and –60.6%, respectively; ptrend<0.001 for all) (Table 3, Figure 3). Of all patients undergoing AVR, the proportion of those aged <75, 75-79, or 80-84 years decreased, and that of patients aged ≥85 years increased (Figure 4).
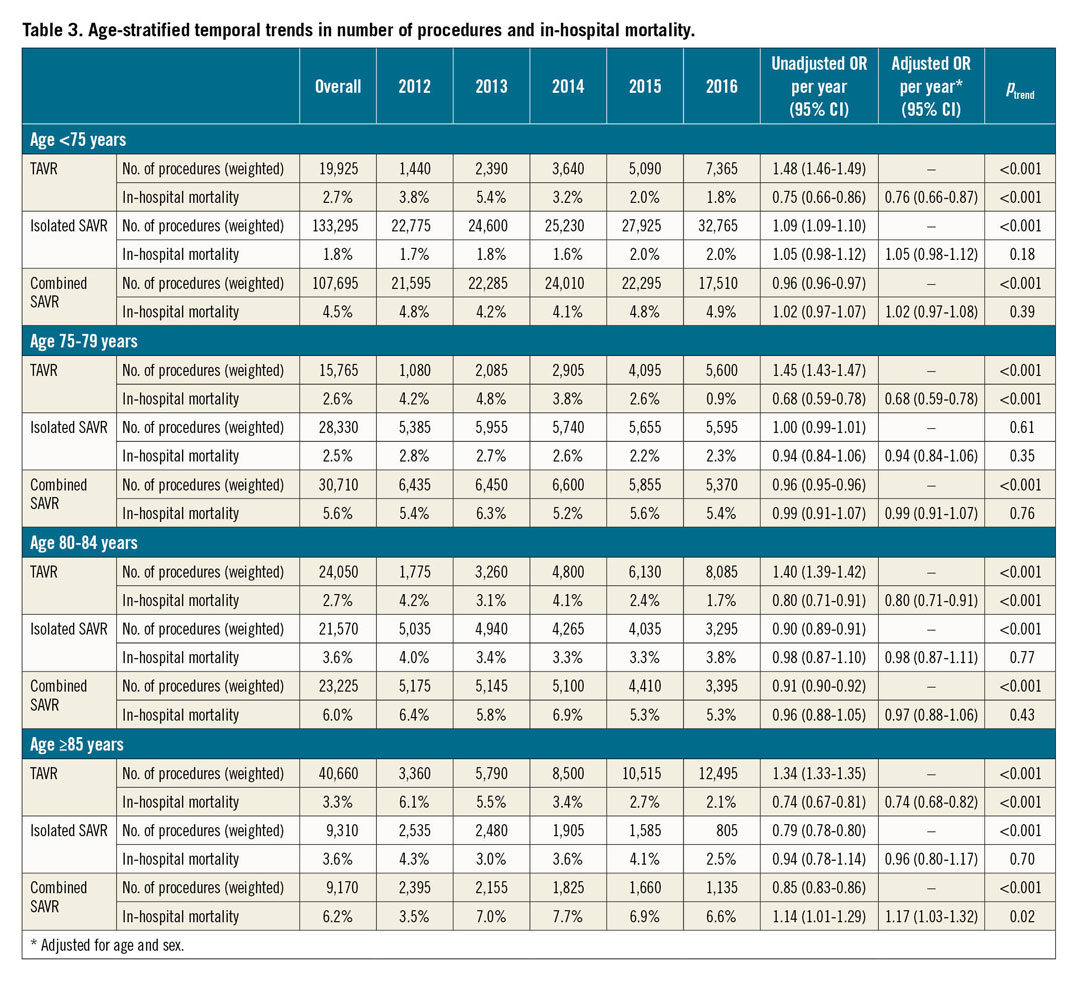
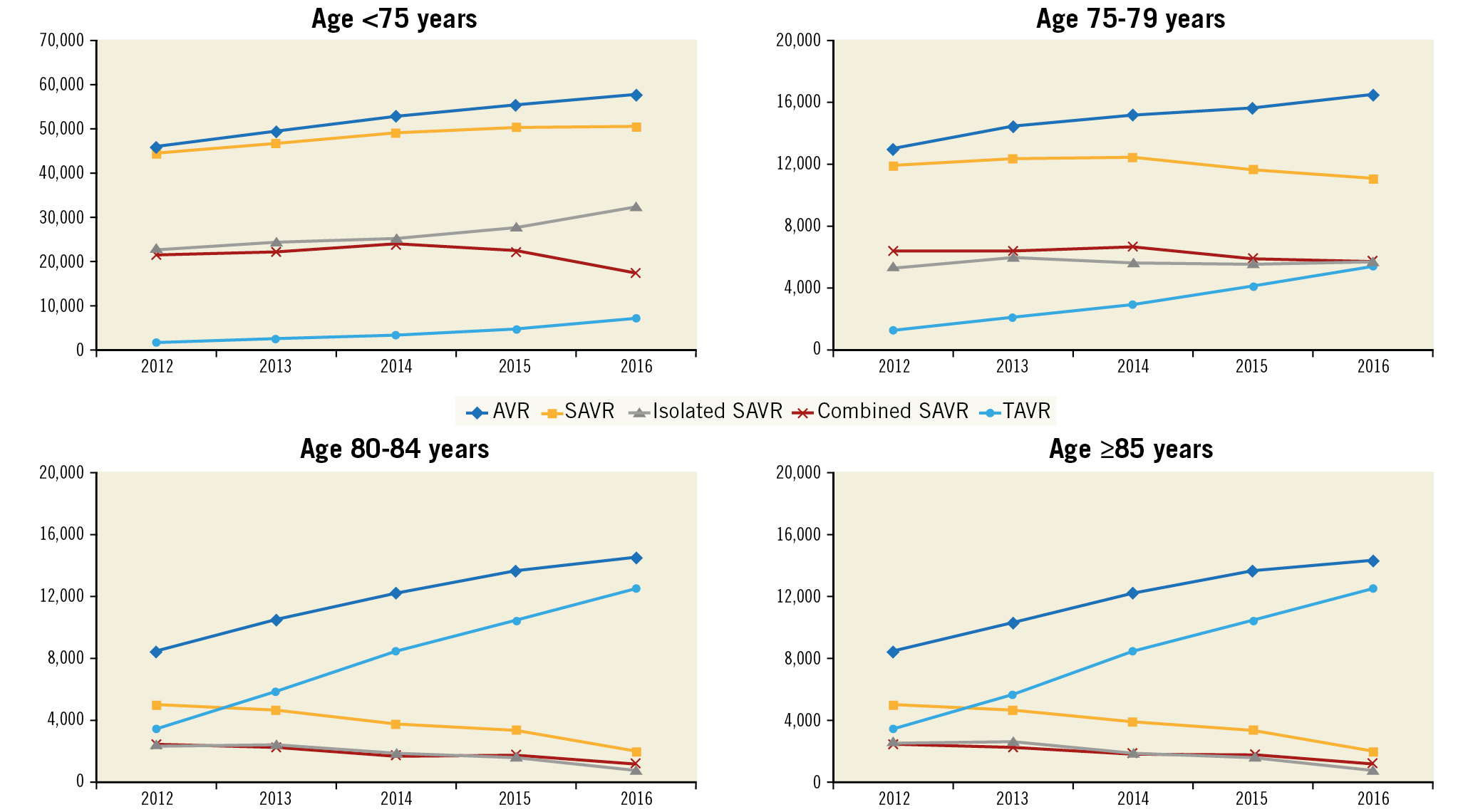
Figure 3. Age-stratified trends in the number of AVRs performed by type from 2012 to 2016. Age <75 years: ptrend<0.001 for all. Age 75-79 years: ptrend<0.001 for all AVR, SAVR, combined SAVR, and TAVR; ptrend=0.61 for isolated SAVR. Age 80-84 years: ptrend<0.001 for all. Age ≥85 years: ptrend<0.001 for all.
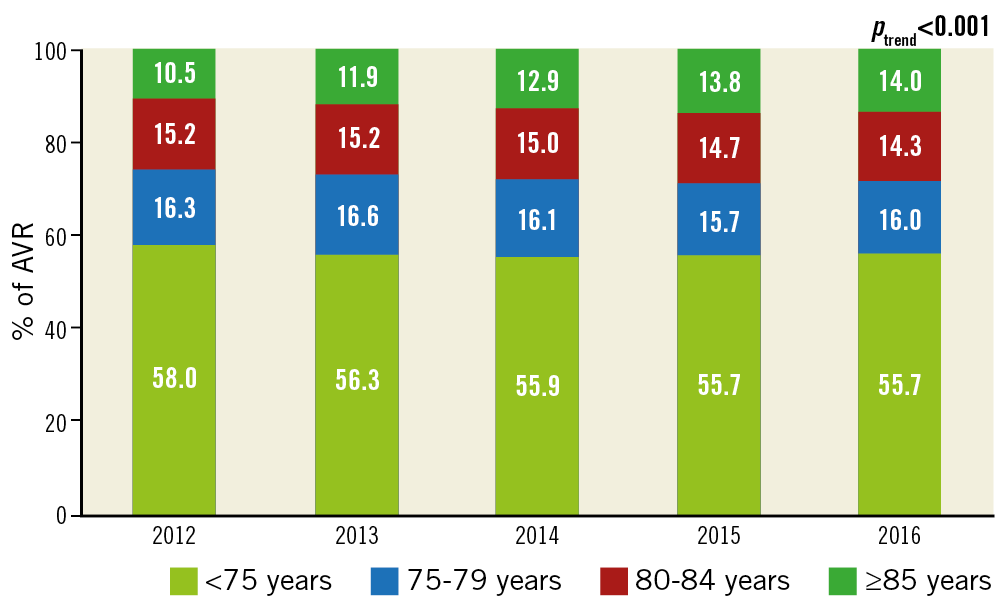
Figure 4. Changes in the age profile of patients undergoing AVR.
IN-HOSPITAL MORTALITY
In-hospital mortality after TAVR decreased over the study period (from 5.0% in 2012 to 1.7% in 2016; aOR 0.75 [0.70-0.79]; ptrend<0.001). In contrast, in-hospital mortality after isolated SAVR (from 2.4% to 2.2%; aOR 1.00 [0.95-1.05]; ptrend=0.96) or combined SAVR (from 5.0% to 5.1%; aOR 1.01 [0.97-1.05]; ptrend=0.66) remained unchanged (Table 2, Figure 5). Similar temporal trends in age- and sex-adjusted in-hospital mortality were seen in the age subgroups (Table 3, Figure 6).
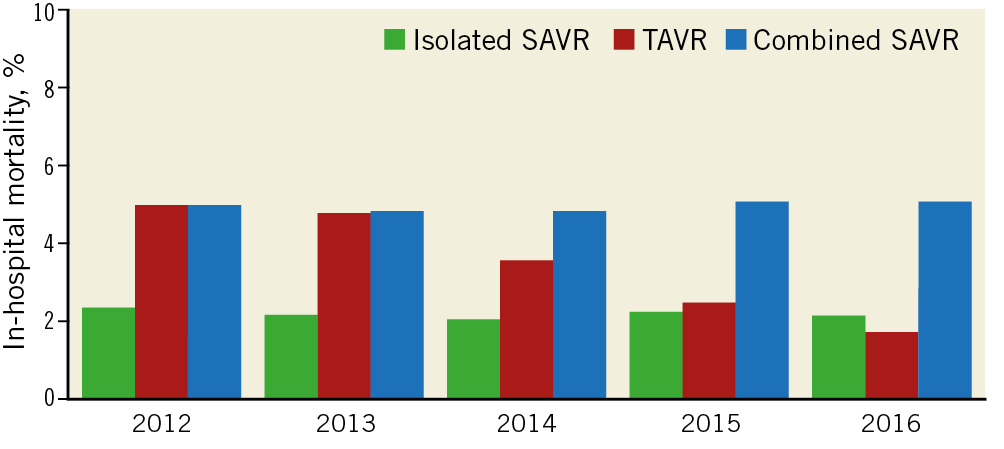
Figure 5. Trends in in-hospital mortality after AVR by type of procedure. ptrend<0.001 for TAVR; ptrend=0.96 for isolated SAVR; ptrend=0.66 for combined SAVR.
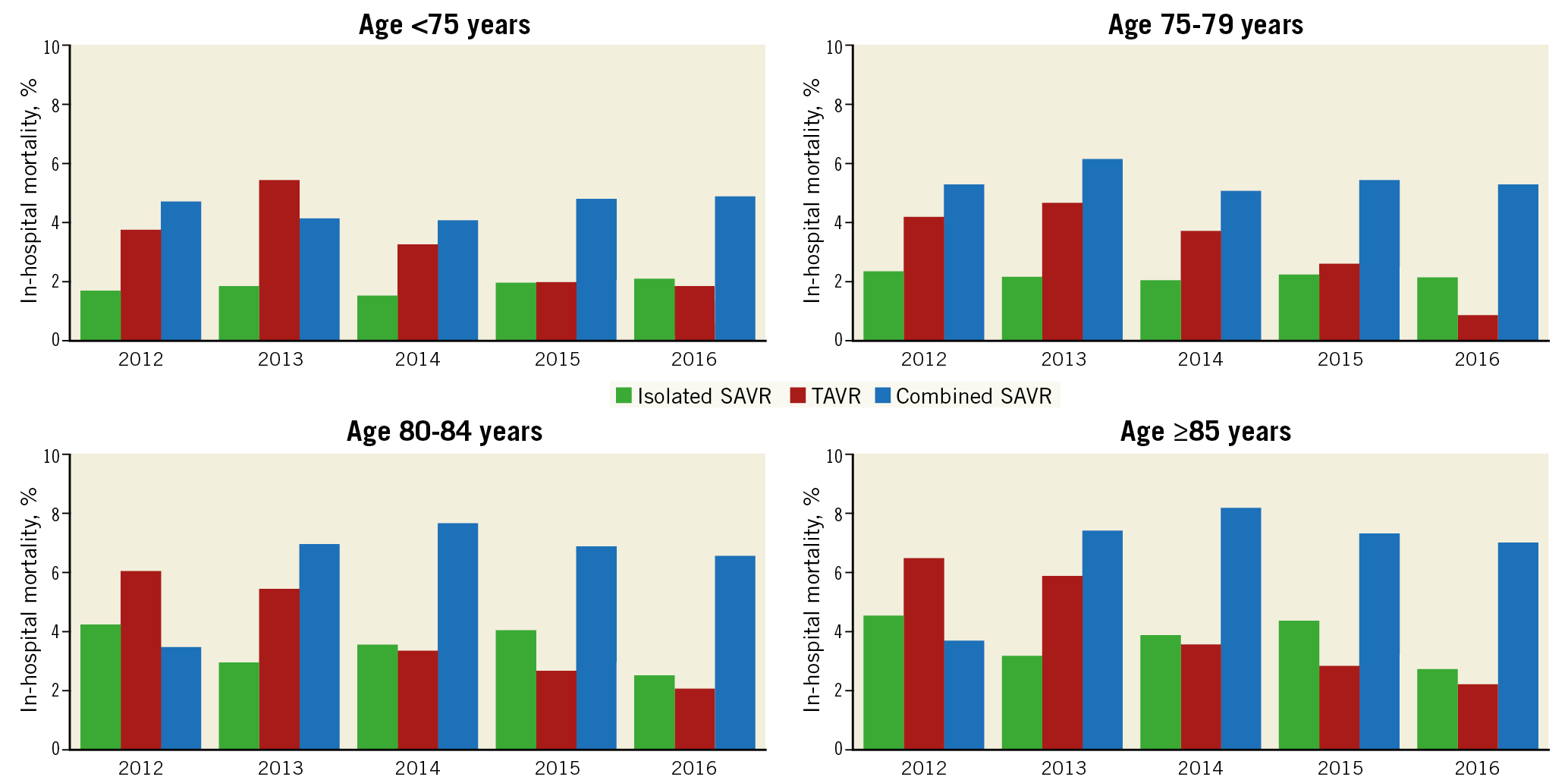
Figure 6. Age-stratified trends in in-hospital mortality after AVR by type of procedure. Age <75 years: ptrend<0.001 for TAVR; ptrend=0.18 for isolated SAVR; ptrend=0.39 for combined SAVR. Age 75-79 years: ptrend<0.001 for TAVR; ptrend=0.35 for isolated SAVR; ptrend=0.76 for combined SAVR. Age 80-84 years: ptrend<0.001 for TAVR; ptrend=0.77 for isolated SAVR; ptrend=0.43 for combined SAVR. Age ≥85 years: ptrend<0.001 for TAVR; ptrend=0.70 for isolated SAVR; ptrend=0.02 for combined SAVR.
Discussion
To our knowledge, our analysis is the most comprehensive assessment of concomitant national trends in utilisation of TAVR and SAVR in the USA. In the present study, we demonstrate an ~30% increase in the number of AVRs performed from 2012 to 2016. It is important to note that the dramatic temporal increase in the number of TAVRs (+338%) was accompanied by a relatively unchanged trend in the number of SAVRs. These results highlight a previously untreated or undertreated population of patients who were not candidates for SAVR but are now being treated with TAVR. These trends were most striking in patients aged ≥85 years in whom AVR increased by ~74%, driven by an almost fourfold increase in the number of TAVRs. Thus, TAVR has addressed an unmet clinical need in these older patients and helpfully complemented SAVR. Our results parallel the recently reported AVR adoption trends in France. Similar to their results, TAVR adoption in the USA lags behind countries such as Germany, where the number of TAVRs had already exceeded isolated SAVRs as of 2014, possibly due to an earlier shift from high- to lower-risk patients6,10.
The NIS, which is an all-payer database, offers a unique opportunity to examine the effect of the adoption of TAVR on SAVR and overall AVR volumes in the USA. Other large administrative or clinical registries such as Medicare or the TVT registry are limited by restriction to Medicare beneficiaries or to TAVR patients only, respectively.
In parallel with these adoption trends, we observed a temporal decrease in age- and sex-adjusted in-hospital mortality after TAVR. This probably reflects the improvements in TAVR technology including delivery system and valve design iterations, use of alternative access in patients with prohibitive vascular anatomy, operator experience, and better patient selection11. In contrast, in-hospital mortality after isolated or combined SAVR remained unchanged during the study period. The temporal trends in SAVR mortality are in contrast to the reported trends in France or Germany where in-hospital mortality after SAVR has declined over time6,10.
Limitations
The NIS is an administrative database; important clinical variables to calculate validated scores such as the Society of Thoracic Surgeons (STS) risk score or logistic EuroSCORE were not available. We did not have information on valve type, procedural success, post-procedural haemodynamics, or paravalvular leak. The U.S. hospital administrative data collection transitioned from ICD-9-CM to ICD-10-CM coding from October 2015 onwards; therefore, the trend data in the number of AVR procedures are subject to being affected by the coding transition. The comorbidity index using ICD-10-CM codes has not yet been developed; therefore, multivariable adjustment for changes in comorbidity burden over time could not be performed. Lastly, NIS is an in-patient database; post-discharge follow-up data are lacking.
Conclusions
The number of AVRs markedly increased in the USA from 2012 to 2016, mainly due to the widespread adoption of TAVR, whereas the number of SAVRs remained relatively stable. The number of SAVRs still exceeded TAVRs by twofold in 2016. In-hospital mortality after TAVR declined, whereas that after SAVR has remained unchanged.
|
Impact on daily practice In this large observational study, we observed a linear increase in the total number of AVRs performed in the USA that was driven mainly by a marked diffusion of TAVR, whereas the number of SAVRs remained relatively stable. The number of TAVRs increased in all age categories. In contrast, the number of SAVRs increased modestly in those aged <75 years and declined in the older age groups. Our results highlight a previously untreated or undertreated population of patients who were not suitable candidates for surgery but are now being treated with TAVR. |
Appendix. Study collaborators
Divyanshu Mohananey, MD, MSc; Medical College of Wisconsin, Milwaukee, WI, USA; Poonam Velagapudi, MD, MS; University of Nebraska Medical Center, Omaha, NE, USA.
Conflict of interest statement
D.L. Bhatt discloses the following relationships – advisory board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. G.C. Fonarow discloses consulting for Abbott and Medtronic. The other authors have no conflicts of interest to declare. The study collaborators have no conflicts of interest to declare.

