Device description
Name and manufacturer: There are two versions of the Advanced Bifurcation Systems (ABS) (ABS, Los Angeles, CA, USA) mother-daughter (MD) platforms: the ABS MD-P (bifurcation stent - provisional), and the ABS MD-Bi (bifurcating stent).
Approval status: Not yet approved for clinical use.
Platform: Stainless steel.
Strut thickness: 105 microns.
Coating: Not coated, available in bare metal only.
Specific stent design: The MD platform consists of two catheters, a mother catheter (MC) and a daughter catheter (DC). On the mother catheter (MC), a mother-daughter stent (MDS) is mounted which is positioned as main branch stent. On the DC, a daughter stent (DS) is mounted in cases where the operator prefers to perform an upfront two-stent strategy (the MD-Bi) or, in cases where the operator prefers the provisional single stenting strategy, there is no stent mounted on the DC (MD-P).
Guiding: 6 Fr, 7 Fr preferred.
Classification according to MADS: The technique is not yet described in MADS (main and side branch simultaneously).
Delivery system: Both the bifurcation and bifurcating stent delivery systems consist of independently movable, dual-balloon catheters. The mother catheter (over-the-wire [OTW]) is mounted with the mother-daughter stent with an aperture through which the daughter catheter (RX) extrudes and leads the system. The mother-daughter stent is fully circumferentially (360 degrees) crimped on the MC, only distal to this aperture. The MDS is crimped partially proximal to the aperture in a fashion such that the shaft of the DC can slide back and forth. The MC has a sleeve proximal to the stent through which the DC is loaded. This sleeve enables advancement of the system on one wire within the guide. In the bifurcating stent delivery system the DC has a stent mounted on the distal half of the balloon.
Stent sizes: Both the MDS and the DS are available in stent sizes ranging from 2.5 mm to 4.0 mm. Every combination between these two is possible.
Stent length: MDS 18 mm, DS 8 mm.
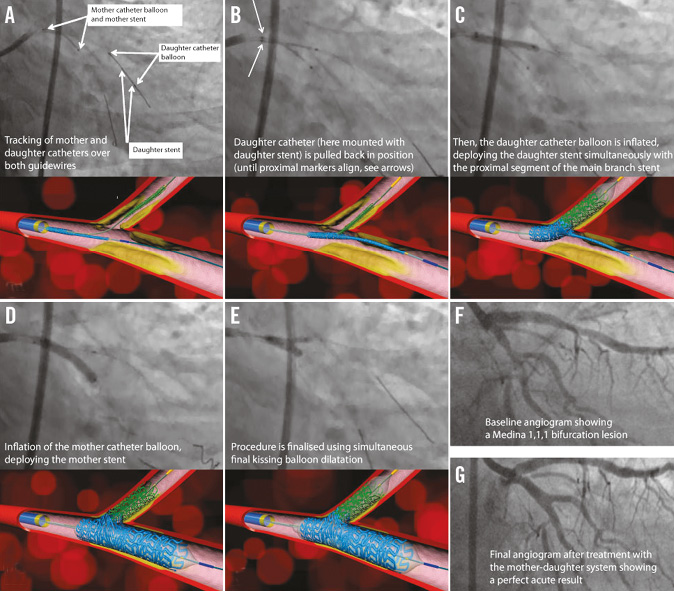
Figure 1. Deployment sequence (left circumflex/obtuse marginal bifurcation).
Procedural details
The system is loaded on the two wires (main and side branch wires) and advanced to the carina, at which point it cannot be advanced any further. The DC which is now in the daughter vessel is pulled back while the MC is held in place. Pullback is completed when the proximal half of the DC is in the proximal segment of the MDS and the two proximal balloon markers are aligned next to each other. The DS which is on the distal half of the DC balloon is now abutting the mother stent, and a bifurcating stent is assembled on the site. The DC balloon is then inflated first which simultaneously deploys the proximal segment of the MDS, as well as the DS, ensuring perfect alignment of the two stents and proper orientation of the proximal segment of the MDS. This automatic alignment is accomplished using the only fixed (non-variable) structure in any bifurcation, namely the carina. This simultaneous deployment and alignment also ensures full tissue coverage. The MD balloon is inflated next, followed by kissing inflation (Moving image 1).
The provisional side branch stenting (MD-P) system consists only of the MD stent. The DC does not have a stent mounted on it. The deployment method is otherwise similar (Moving image 2). The deployment sequence is demonstrated in Figure 1.
Clinical data
FIRST-IN-MAN (FIM)
Patients with de novo bifurcation lesions were enrolled in a pilot, non-randomised, multicentre trial evaluating the safety, technical feasibility, and acute efficacy of the ABS platform1. The primary endpoint was 30-day composite MACE (death, MI, and target vessel revascularisation). Secondary endpoints were procedural success and safety at six months. Angiographic follow-up was performed at six months in seven patients. IVUS imaging was performed in a subset (Moving image 1 - Moving image 13).
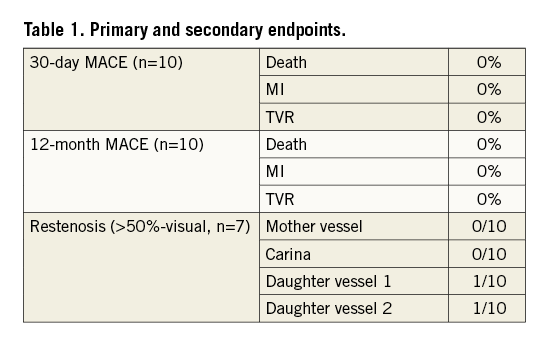
A total of 10 patients were enrolled. The MD-Bi (n=7) was deployed in the following bifurcations: left anterior descending (LAD)-diagonal (Diag) (n=2), left circumflex (LCx)-obtuse marginal (OM) (n=3), and posterior descending artery (PDA)–postero-lateral branch (PLB) (n=2). The MD-P (n=3) was deployed in LAD-Diag only. All lesions (both groups) were Medina 1,1,1 by visual estimation. Average patient age was 55. The 30-day composite MACE was 0%. Delivery rate and procedural success was 100%, and there was no residual stenosis by IVUS, which revealed good stent apposition with complete coverage of the carina.
Twelve-month composite MACE as well as TVR has remained at 0%. All patients are free of angina. Angiographic follow-up to date has been completed in seven patients and has revealed edge restenosis in two side branch vessels only (Table 1).
Ongoing studies
A sirolimus-coated version of the systems is under development and human studies including the LM are in the planning stages.
Unique features
– The only system available with simultaneous side branch and (proximal) main branch stent deployment designed for perfect alignment of the side branch stent without leaving a gap or having an overlap at the side branch ostium.
– The hybrid monorail/OTW system minimises the chances of wire-wrap and permits easier handling of the device. The system can be loaded on the daughter catheter first and advanced to the point where the DC is in the coronary artery and the MC is just proximal to the tip of the guide catheter. The mother vessel can then be wired through the OTW lumen of the MC to avoid wire-wrap.
– Provisional side branch stenting system is designed such that, should the side branch require a stent, there would be no gap in tissue coverage while access is guaranteed.
Potential improvements
Sirolimus coating with biodegradable polymer.
Conflict of interest statement
M. Khorsandi, S. Kar and H. Bourang have an equity interest in ABS. The other authors have no conflicts of interest to declare.
Moving image legends
Moving image 1. Bifurcating stent deployment.
Moving image 2. Bifurcating stent deployment (provisional side branch stenting).
Moving image 3. Bifurcating stent implant - LCx OM baseline angio.
Moving image 4. Bifurcating stent implant - LCx OM after predilatation.
Moving image 5. Bifurcating stent implant - LCx OM daughter in branch vessel.
Moving image 6. Bifurcating stent implant - LCx OM daughter catheter pullback.
Moving image 7. Bifurcating stent implant - LCx OM system assembled at the carina (1 of 2).
Moving image 8. Bifurcating stent implant - LCx OM system assembled at the carina (2 of 2).
Moving image 9. Bifurcating stent implant - LCx OM daughter balloon inflation.
Moving image 10. Bifurcating stent implant - LCx OM mother balloon inflation.
Moving image 11. Bifurcating stent implant kissing inflation.
Moving image 12. Bifurcating stent implant - LCx OM final angio 1.
Moving image 13. Bifurcating stent implant - LCx OM rotational final angio 2.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Bifurcating stent deployment.
Moving image 2. Bifurcation stent deployment (provisional side branch stenting).
Moving image 3. Bifurcating stent implant - LCx OM baseline angio.
Moving image 4. Bifurcating stent implant - LCx OM after predilatation.
Moving image 5. Bifurcating stent implant - LCx OM daughter in branch vessel.
Moving image 6. Bifurcating stent implant - LCx OM daughter catheter pullback.
Moving image 7. Bifurcating stent implant - LCx OM system assembled at the carina (1 of 2).
Moving image 8. Bifurcating stent implant - LCx OM system assembled at the carina (2 of 2).
Moving image 9. Bifurcating stent implant - LCx OM daughter balloon inflation.
Moving image 10. Bifurcating stent implant - LCx OM mother balloon inflation.
Moving image 11. Bifurcating stent implant kissing inflation.
Moving image 12. Bifurcating stent implant - LCx OM final angio 1.
Moving image 13. Bifurcating stent implant - LCx OM rotational final angio 2.