
Abstract
Aims: The aim of this study was to evaluate the safety and performance of renal nerve stimulation (RNS) for diagnostic mapping of the renal nerves.
Methods and results: In this first-in-man study, twenty hypertensive patients underwent RNS using the ConfidenHT system. Bilateral stimulations were performed at three to four sites per artery at 2 and 4 mA. The primary endpoint was change in systolic blood pressure (SBP). Mean office blood pressure was 156/89 mmHg. No periprocedural adverse events occurred. Stimulation with 2 mA resulted in a maximum change of 8.3±6.3 mmHg in SBP (based on 119 stimulations; p<0.001), while stimulating with 4 mA resulted in a maximum change of 10.1±7.8 mmHg (based on 61 stimulations; p<0.001). The mean change in SBP did not vary between mid, distal or branch sites when stimulating at 2 mA but was significantly higher at ostial (23±14 mmHg) than at non-ostial locations (9±7 mmHg) when stimulating at 4 mA (p=0.003).
Conclusions: RNS can be performed safely and effectively along the renal artery and results in a large variation in temporary BP changes per patient and per anatomic location. RNS might help in optimising treatment effect and selecting potential responders to renal sympathetic denervation.
Abbreviations
BP: blood pressure
DBP: diastolic blood pressure
HR: heart rate
MAP: mean arterial pressure
RDN: renal sympathetic denervation
RNS: renal nerve stimulation
SAE: serious adverse events
SBP: systolic blood pressure
SNS: sympathetic nervous system
Introduction
Hypertension forms a major health problem worldwide associated with a significantly increased risk of cardiac and cerebrovascular events1,2. While the effect of pharmacological treatment is accepted, a large proportion of patients remains uncontrolled due to non-adherence, side effects and/or failure to reach blood pressure (BP) targets despite maximum tolerated regimens3,4. Several novel device-based treatment strategies were recently introduced to help in controlling BP by modulating the sympathetic nervous system (SNS)5. Among these, renal denervation (RDN) has been the most widely studied. When performing RDN, one of the major challenges is the lack of landmarks along the renal arteries identifying the exact location of the renal nerves. The treatment itself remains “blinded” as no intraprocedural feedback or guidance is provided on which patient will benefit or on whether technical success is achieved. The importance of the latter was demonstrated by more recent studies showing non-response rates of up to 37%, mostly attributed to inadequate patient selection and incomplete denervation6,7.
The ConfidenHT™ system (Pythagoras Medical Ltd, Herzliya, Israel) is an add-on technology for RDN, allowing mapping of the renal nerves with the objective of identifying potential responders and providing periprocedural guidance. The aim of the present study was to demonstrate the safety and performance of RNS using the ConfidenHT system in stimulating the renal sympathetic nerves in patients with hypertension.
Methods
STUDY DESIGN
The present study is a prospective, feasibility, open-label, single-arm, multicentre study (three European sites: Hippokration Hospital, Athens, Greece; Erasmus Medical Center, Rotterdam, the Netherlands; and Utrecht Medical Center, Utrecht, the Netherlands). The ConfidenHT system was used for diagnostic mapping of the renal nerves through electrical renal nerve stimulation (RNS). Clinical follow-up visits were scheduled at 30 days and three months post procedure. The study protocol was approved by local ethics committees (ClinicalTrials.gov: NCT02777216; approval IRB MEC-2016-542 for the Netherlands and 167CLP for Greece). All patients signed written informed consent.
STUDY POPULATION
Patients were included when the following criteria were met (N=20): age >18-75 years with hypertension (office SBP ≥140 mmHg or diastolic blood pressure [DBP] ≥90 mmHg), potential candidates for RDN or a planned elective cardiac catheterisation, glomerular filtration rate (eGFR) >45 ml/min and a main renal artery with a diameter ≥4.0 mm. Patients were excluded when there was relevant renal artery disease (renal artery stenosis of >30%, aneurysm or fibromuscular dysplasia), a history of RDN or renal artery stenting, triple ipsilateral renal artery ostia, known secondary causes of hypertension, diabetes mellitus type 1 or an active implantable medical device (e.g., implantable cardioverter-defibrillator [ICD] or cardiac resynchronisation therapy device [CRT-D]; baroreflex stimulator).
STUDY MEASUREMENTS
Prior to the procedure and during follow-up visits, office BP was measured and laboratory measurements (creatinine) were performed. Serious adverse events (SAE) and adverse events (AE) were reported spontaneously by the subject or observed at regular follow-up, or any time in between, and were to be recorded by the investigator. An SAE was defined as an AE that led to death, or led to serious deterioration in the health of the subject that resulted in either 1) a life-threatening illness or injury, or 2) a permanent impairment of a body structure or a body function, or 3) inpatient or prolonged hospitalisation, or 4) medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function. Device-related deficiencies were also reported. The primary safety outcome was defined as the occurrence of SAE (system- and/or procedure-related events). The primary performance outcome was the change in arterial BP in response to RNS.
STUDY PROCEDURE
All patients were preloaded with 300 mg aspirin, if naïve, and advised to continue with aspirin for at least one month. Preprocedurally, 100 IU heparin/kg was administered to achieve an activated clotting time >250 s. In 17 out of 20 patients the procedure was performed under local anaesthesia. Midazolam (up to 15 mg) and fentanyl (125 µg) were used only in case of discomfort (Table 1). The remaining three procedures were performed under general anaesthesia using propofol and remifentanil or alfentanil. After administration of local or general anaesthesia, common femoral artery access was achieved according to local clinical practice and an 8 Fr sheath was then introduced. In case of scheduled RDN (N=7), either the Symplicity Spyral™ multielectrode catheter (N=6) (Medtronic, Minneapolis, MN, USA) or the EnligHTN™ ablation catheter (N=1) (St. Jude Medical, St. Paul, MN, USA) was used according to standard instructions for use.
The ConfidenHT system consists of two main parts, the console and the catheter (Figure 1). The console delivers electrical energy to the catheter using a multichannel stimulator, which produces controlled electrical stimulation during the mapping procedure. The console independently controls each electrode on the stimulation catheter. The following stimulation parameters were pre-programmed: stimulation amplitude, frequency, pulse duration and stimulation duration. During stimulation, arterial BP and MAP were continuously measured invasively through a side port of the guiding catheter manifold and analysed and displayed on the console. The ConfidenHT catheter consists of a flexible catheter shaft, catheter tip and an ergonomic handle with a wheel to open and close the basket. The monorail catheter is compatible with an 8 Fr guiding catheter and a 0.014” guidewire. The catheter distal end is composed of an expandable non-occlusive nitinol basket with four peripheral radiopaque stimulation electrodes, mounted on each basket’s strand. The basket size when expanded is 8 mm (Figure 1).
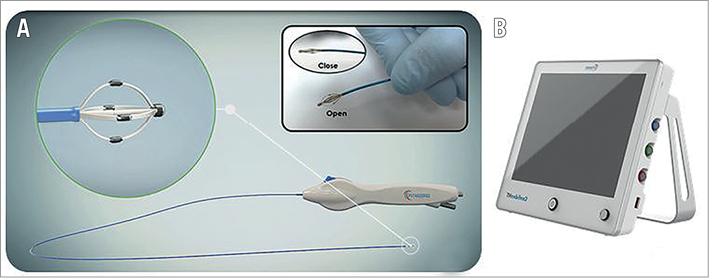
Figure 1. The ConfidenHT system. The ConfidenHT catheter consists of a flexible catheter shaft, catheter tip and an ergonomic handle with a wheel to open and close the basket (A). The ConfidenHT console delivers electrical energy to the ConfidenHT catheter using a multi-channel stimulator (B).
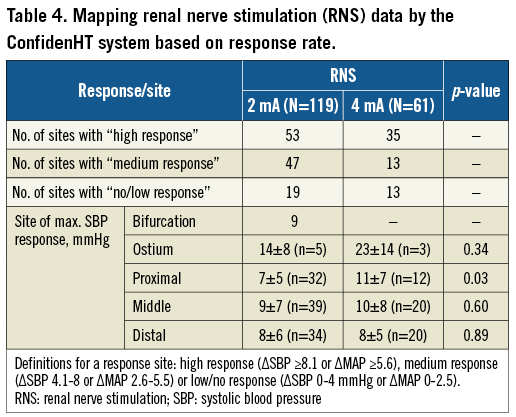
The ConfidenHT system’s principal concept is based on monitoring of haemodynamic response elicited by site-specific electric stimulation in order to map the location of the renal nerves. Electrical stimulation of sympathetic renal nerves induces a sympathetic response by activating afferent and efferent nerve pathways which in turn drives a transient change in several physiological measurable parameters such as heart rate (HR) and BP8. Stimulations were performed for 120 s each in the left and right renal arteries, including branches at three to four locations per artery (branches, distal, mid and proximal) at a frequency of 20 Hz. In all anatomical sites, stimulations were performed at 2 mA (n=119), while in some anatomical sites additional stimulation with 4 mA was performed (n=61). Each stimulation generated an electrical signal in all four electrodes (configuration as in Figure 1) for the given duration. Changes in arterial BP response and HR were continuously monitored and recorded during each stimulation. For analytical purposes, arbitrary response thresholds were defined for the change in SBP and MAP. The thresholds used to categorise the rate of response were: ΔSBP ≥8.1 mmHg or a ΔMAP ≥5.6 mmHg for high response, ΔSBP 4.1-8 mmHg or ΔMAP 2.6-5.5 mmHg for medium response, or ΔSBP 0-4 mmHg or ΔMAP 0-2.5 mmHg for low/no response.
The duration of the procedure was defined as the time interval between inserting the RNS catheter and the time when the catheter was retrieved outside the body of the patient.
STATISTICAL ANALYSIS
All measured variables and derived parameters were listed individually and, if appropriate, summarised using descriptive statistics. Categorical variables were expressed in absolute, relative frequencies and percentages. Continuous variables were expressed as mean±standard deviation (SD). The incidences of SAE are presented with percentages and 95% CI. Continuous variables were compared using the Student’s t-test. All statistical tests were two-tailed. A p-value <0.05 was considered statistically significant. Statistical analysis was performed using SAS, version 9.3 (SAS Institute, Cary, NC, USA).
Results
A total of 20 patients were enrolled. Baseline characteristics are presented in Table 2. In brief, nine patients were male and mean age was 60±11 years. Mean duration of antihypertensive treatment was 6.7 years.
SAFETY OUTCOME
No periprocedural adverse events occurred. No signs of angiographically visible spasms/thrombus or dissection were observed post procedure (Figure 2). No severe adverse events were reported at 30-day follow-up (N=20). Device-related events occurred in 1/20 patients (reported as myalgia in the back), and procedure-related events occurred in 3/20 patients (Table 3). All device- and/or procedure-related events were resolved at three-month follow-up. Renal function remained unchanged during follow-up (baseline eGFR 81±19 ml/min vs. 78±16 ml/min at 30 days (p=0.17), and 78±17 ml/min at three months (p=0.43).
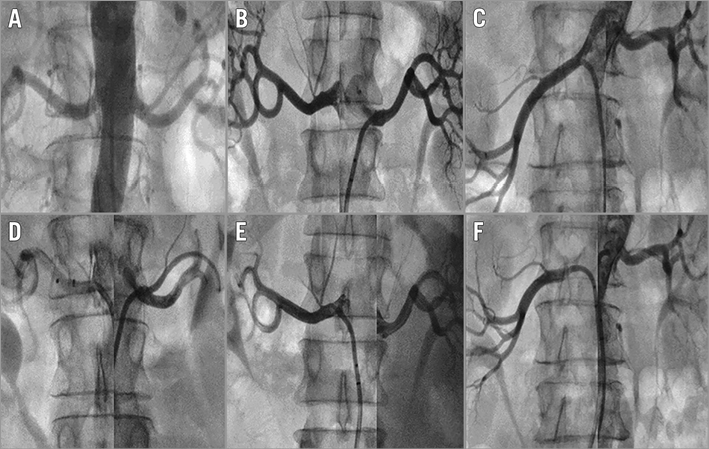
Figure 2. Renal angiograms pre and post RNS using the ConfidenHT system. No signs of angiographically visible spasms/thrombus or dissection were observed post renal nerve stimulation. A) - C) Pre procedure. D) - F) Post procedure.
PERFORMANCE OUTCOME
A total of 194 stimulations were performed: 119/194 stimulations were performed with the 2 mA amplitude and 61/194 with the 4 mA. The remaining 14/194 stimulations had different amplitudes in the range of 2 mA to 10 mA, which were performed in the first three patients in order to set the stimulation amplitude to either 2 mA or 4 mA. An average of 9.7 stimulations/patient was performed.
Response to electrical stimulation varied among different anatomic locations within the arteries as well as between patients (Figure 3). The mean change in SBP in the overall study population was 9±7 mmHg, with a maximum change in SBP of 34 mmHg. The mean change in MAP in the overall study population was 6±5 mmHg, with a maximum change in MAP of 26 mmHg. The mean change in SBP per patient varied between 4 and 21 mmHg, while the mean change in MAP varied between 3 and 14 mmHg (Figure 4).
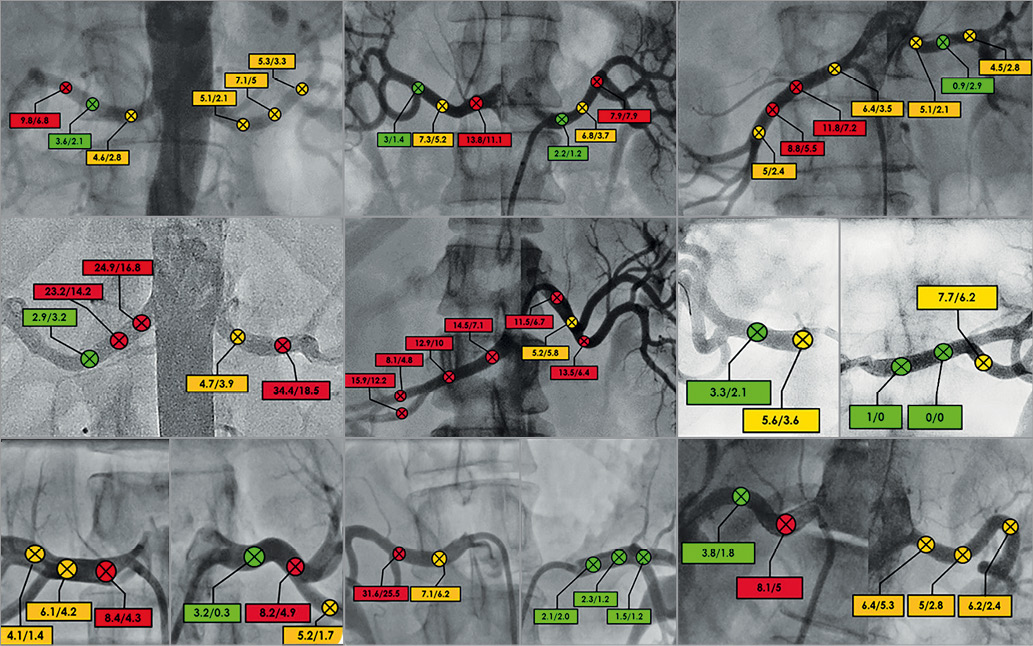
Figure 3. Response variation between different anatomical locations per patient in delta SBP and delta MAP. Definitions for a response site (ΔSBP/ΔMAP): red=high response (ΔSBP ≥8.1 or ΔMAP ≥5.6), orange=medium response (ΔSBP 4.1-8 or ΔMAP 2.6-5.5) or green=low/no response (ΔSBP 0-4 mmHg or ΔMAP 0-2.5). MAP: mean arterial pressure; SBP: systolic blood pressure
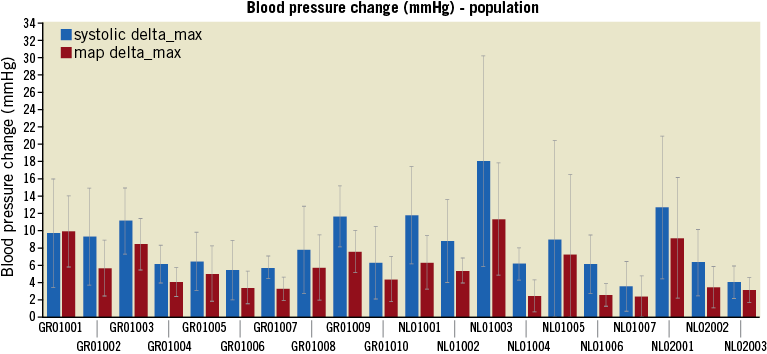
Figure 4. BP change within patients induced by renal nerve stimulation (RNS). Change in SBP and MAP due to RNS per patient, demonstrating different responses to stimulation. Patients 1-17: local anaesthesia, patients 18-20: general anaesthesia.
A high response (ΔSBP ≥8.1 mmHg or ΔMAP ≥5.6 mmHg) to stimulation with 2 mA was observed in 53 sites with a mean change in SBP of 13±6 mmHg: medium response (ΔSBP 4.1-8 mmHg or ΔMAP 2.6-5.5 mmHg) was observed in 47 sites with a mean change in SBP of 5±2 mmHg and low/no-response (ΔSBP 0-4 mmHg or ΔMAP 0-2.5 mmHg) was seen in 19 sites with a mean change in SBP of 2±2 mmHg. Procedural data on the RNS sites and numbers with both 2 mA and 4 mA are summarised in Table 4. Per-patient analyses showed a lack of high response with 2 mA to any of the stimulations in 1/20 patients. All other patients showed a high response to one of the stimulations at least once. Overall, the maximum change in SBP to 2 mA stimulation was 8±6 mmHg, while stimulating with 4 mA resulted in a maximum change in SBP of 10±8 mmHg (p=0.09). Distribution of the stimulations along the renal arteries was as follows: 47/194 (24%) at the ostium/proximal part of the artery, 54/194 (28%) at the middle part, 51/194 (26%) at the distal part, and 42/194 (22%) at the branches. No significant change was observed in SBP response between proximal, mid, distal and branch locations at 2 mA (p=0.77), while stimulation with 4 mA showed a significant difference in SBP response between ostial (23±14 mmHg) and non-ostial locations (9±7 mmHg), p=0.003.
No difference was found in the response to stimulation in patients who did or did not undergo subsequent RDN. The change in SBP due to mapping at 2 mA in patients who underwent RDN was 8.4±8.0 mmHg versus 8.2±5.0 mmHg in the remaining patients (p=0.91).
Because 40% of the patients used beta-blockers, which might have influenced BP response during stimulation, a sub-analysis was performed in patients with versus those without beta-blockers. SBP change in patients without beta-blockers was 8.6±6.2 mmHg versus 7.7±6.4 mmHg in patients with beta-blockers (p=0.48) when stimulations were performed at 2 mA, versus 9.2±9.1 mmHg versus 11.1±6.0 mmHg (p=0.34), respectively, when stimulations were performed at 4 mA.
The average time to maximal response was 45 seconds. Out of the 20 patients, one subject withdrew informed consent two months post procedure for personal reasons and was considered as lost to follow-up at the three-month visit.
PROCEDURE
The mean amount of contrast used during the procedure was 174.0±69.7 ml. The mean fluoroscopy time was 12±6 minutes. Mean time of the mapping procedure was 44±11 minutes. Mean total procedure time was 63±20 minutes.
Discussion
This feasibility study demonstrated that RNS using the ConfidenHT system is safe and effective along the renal artery. A large variation in temporary BP changes was observed per patient and per anatomic location within the artery in response to RNS, suggesting that it may be used to identify anatomic areas for effective RDN.
Previous work has shown that RDN can contribute to better BP control; however, there are still a number of patients who do not seem to benefit from the treatment9,10. This leaves an unmet need in terms of identifying potential non-responders11. Next to patient selection, the blind nature of the RDN procedure has been hypothesised to be one of the main reasons for non-response12,13. A way to convert the blind nature of the procedure in targeted therapy is to identify the locations along a renal artery at which a patient’s BP reacts to RNS. The concept was first introduced in pathophysiological work from Chinushi et al who demonstrated that electrical stimulation of the renal nerves in the proximal renal arteries can provoke action potentials and a change in BP14. These findings were taken further by Lu et al who published the feasibility of targeted RDN guided by RNS in the proximal renal artery, resulting in both BP reduction and sympathetic inhibition as measured by plasma norepinephrine levels8. In addition, both animal studies support basic evidence that renal sympathetic nerve locations are distributed unequally between different animals and anatomic locations along a particular renal artery8,14,15. More recently, two small pivotal in vivo studies demonstrated that the BP response to RNS could be blunted significantly following RDN, hypothesising that RNS could even serve as an endpoint for RDN16,17. The first study by Gal et al investigated the feasibility of RNS in eight patients with resistant hypertension undergoing RDN and showed that BP increased significantly during RNS (from 108/55 mmHg to 132/68 mmHg; p<0.001) with stimulation outputs of 10 mA and 20 mA. After RDN, the maximum BP response to RNS was blunted significantly (from +43 mmHg to +9 mmHg after RDN, p<0.01)16. The second study, performed by de Jong and co-workers, described the association between RNS pre and post RDN and change in ambulatory BP measurement at three to six months post RDN. The authors described a correlation between the change in RNS-induced BP change pre versus immediately post RDN and the change in ambulatory blood pressure monitoring (ABPM) at follow-up, for both SBP (R=0.77, p<0.001) and DBP (R=0.79, p<0.001). However, in both studies RNS was performed using either a conventional quadripolar EP-XT catheter (C.R. Bard, Inc., Murray Hill, NJ, USA) which only allowed stimulation with a single electrode at the tip of the catheter or a reprogrammed version of the multielectrode basket ablation catheter (EnligHTN) which is no longer on the market.
To the best of our knowledge, the ConfidenHT system is the first dedicated RNS system and has proved, in the present study, to be capable of successfully identifying locations with positive SBP change during RNS. We found a large variability in the change in BP between different stimulated locations and between patients, in line with previous work8,14. More specifically, low or no change of BP to any of the stimulations at either 2 or 4 mA (<4 mmHg in SBP was found in up to 30% of patients in the present study) was also reported in previous studies16,17. The estimation of the non-responders based on arbitrary RNS thresholds in this study is in line with the previously published results of several RDN studies, reporting non-response rates between 8% and 37%7.
The highest BP change was observed in the ostial part when comparing stimulation with 2 mA and 4 mA; however, probably due to low stimulation numbers, the change did not reach statistical significance. Stimulation with 4 mA showed a significant difference in SBP response between ostial and non-ostial locations. The latter supports other pathophysiological studies, demonstrating that renal nerves at the ostial sites are located more distally from the arterial lumen as compared to more distal locations15, such that higher energy is needed for effective RNS in these sites.
With the potential of RNS reported above, the change in SBP to electrical stimulation could be used as a diagnostic tool to identify patients with a high likelihood of response. In addition, responsiveness to RNS at different locations may indicate treatment targets along the renal artery, with the potential to identify and increase the technical success of RDN.
Little is known of the relationship between stimulation or ablation of the nerves travelling along the renal artery lumen and pain. In 1975, DeWolf and Fraley described that stretching of the renal capsule, pelvis, artery, or vein or distension of the pelvis or upper ureter produces the sensation of pain18. Of note, in a previous RNS study17, all procedures were performed under general anaesthesia that could have confounded the effects on sympathetic tone and BP response. However, the authors successfully stimulated 105 sites, whereas 78/105 sites (74.3%) showed >10 mmHg SBP increase. Here, we studied three patients under general anaesthesia who demonstrated the same variability in response between patients and within different renal loci in the same patient, as seen in those studied under local anaesthesia, which further mitigates the potential lack of response due to general anaesthesia.
Limitations
Our study has several limitations. First, the present first-in-man report included 20 patients and therefore larger studies with adequate statistical power to confirm the usability of RNS are warranted. Second, the duration of renal nerve stimulation (120 s per stimulation at both 2 and 4 mA) resulted in an average mapping time of 44 minutes. Given the findings of our study, this could be reduced substantially in the future. At present, a second-generation ConfidenHT catheter is under development incorporating the ability to perform RDN. Subsequent studies are needed to identify the feasibility of the device to determine treatment response. Third, temporal changes to stimulation at 2 and 4 mA were assessed. The catheter was not moved in between stimulations at 2 and 4 mA at the same spot, providing a reasonable level of assurance that mapping was performed at the exact same location. Nevertheless, we cannot be sure that minimal changes in catheter position might not have occurred.
Conclusions
In the present study we demonstrated that the ConfidenHT system constitutes a safe and effective method to achieve RNS. Diverse temporary BP changes were observed per patient and per anatomic location within the artery in response to RNS. These results suggest that the ConfidenHT system may be used to identify renal nerve loci with better RNS-induced BP response in order to achieve effective RDN and help in identifying responders to RDN.
| Impact on daily practice The change in SBP to electrical stimulation could be used as a diagnostic tool for an appropriate patient selection for RDN. In addition, responsiveness to RNS at different locations may indicate treatment targets along the renal artery with the potential of identifying and increasing the technical success of RDN. |
Funding
The sponsor of the study, Pythagoras Medical, was responsible for the selection of clinical sites, as well as data management.
Conflict of interest statement
K. Tsioufis has received grants or honoraria from Medtronic, St. Jude Medical, Bayer, Novartis, AstraZeneca, Boehringer Ingelheim, Pfizer, Chiesi, Pharmanel, Sanofi, Vianex, Win-Medica, ELPEN, Recordati, Servier and Pythagoras. K. Dimitriadis has received a research grant or honoraria from Medtronic and Pythagoras. D. Konstantinidis has received a research grant from Medtronic. D. Tousoulis has received a research grant or honoraria from Medtronic and Pythagoras. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie, Deutsche Hochdruckliga und Deutsche Forschungsgemeinschaft (SFB TRR 219), and has received study grants and lecture fees from Medtronic and Recor. J. Daemen has received institutional research support from Abbott Vascular, Medtronic, Boston Scientific, Acist and Pie Medical as well as speaker and consultancy fees from Medtronic, Acist, and Pythagoras. The other authors have no conflicts of interest to declare.

