- optical coherence tomography
- safety
- efficacy
- frequency domain
Abstract
Aims: To determine the safety and efficacy of frequency domain OCT, which can scan at much higher rates and make it possible to avoid an occlusion balloon and image during an angiographic injection through guide catheter. The catheters have diameters ranging from 2.7 to 3.5 Fr. The presence of the imaging catheter increases fluid resistance to the injection of viscous solutions necessary for clearing the blood.
Methods and results: The Volcano 3.5 Fr frequency domain OCT catheter system was investigated for safety in (a) n=10 porcine studies using acute and 30-day histology, and (b) for efficacy in n=9 in vivo porcine coronary arteries. We found: (a) frequency domain imaging is safe in the porcine model using histology as an endpoint; (b) the addition of a viscous contrast (iodixonal) to saline is superior for lumen clearance compared to saline alone; (c) hand injection, 4 ml/sec, and 6 ml/sec power injection all provided similar vessel wall clearance; (d) the anticipated loss of vessel wall visualisation with left main injection (due to half the injectate in the non-imaged vessel) was not evident in proximal and middle coronary artery OCT catheter positions.
Conclusions: Frequency domain OCT is safe and efficacious in the porcine model.
Introduction
The initial clinical use of OCT time domain systems required balloon occlusion of the coronary artery to clear red blood cells (RBCs)1-4, although more recent studies have abandoned the balloon occlusion technique5,6. Early clinical trials that utilise frequency domain systems (which include spectral domain, optical frequency domain imaging [OFDI], and Fourier domain systems) are currently underway, and never require balloon occlusion of the coronary artery to accomplish blood clearance7-10. The newer frequency domain systems utilise catheters that have greater diameters than those utilised with time domain (Lightlab Imaging time domain 0.019 inches versus frequency domain catheters, which range from 2.7 to 3.5 Fr11,12). This translates to an average diameter for frequency domain OCT catheters of 0.87 to 1.05mm, which is a third of the diameter of a 3mm coronary artery. Increased resistance to the injection of blood clearing solutions is anticipated. Further, due to “transfer of displacement energy”, solutions of greater viscosity are desired to both move RBCs out of the imaging field, and delay RBCs’ reappearance in the imaging field, only compounding this problem. The impact of increased resistance to injection on safety and efficacy has not been examined in a systematic approach to date.
Competing techniques to clear RBCs during frequency domain imaging include power versus hand injection12,13, and high versus lower viscosity solutions14,15. To examine the impact of these alternatives on safety endpoints, power flushing in combination with 3.5Fr frequency domain OCT catheters was performed in porcine coronary arteries, and these animals sacrificed acutely and at 30days, to examine the impact on histology. To determine the most efficacious approach to obtain arterial wall visualisation, randomised studies of power injection versus hand injection, saline versus iodixonal (Visipaque; GE Healthcare, Princeton, NJ, USA), and different power injection rates, were also examined in the invivo porcine coronary artery.
Methods
OCT instrumentation
A prototype Volcano OCT imaging system was used for recording images. The system is based on a swept-laser with 100 nm bandwidth, 1,310nm centre wavelength, and repetition rate of 20kHz. The system provides an OCT scan diameter of approximately 7mm after compensating for refractive index of contrast media. An optical power of 3mW is incident on the arterial wall. Catheter rotation speeds of 1,200 or 1,800 rpm were used, yielding frame rates of 20 or 30fps and circumferential line densities of 1,000 or 667 A-scans/frame. These technical changes were due to evolution of the Volcano OCT technology during these studies, although they would not be associated in changes in image quality. No pullbacks were performed for the purposes of this study, with the OCT catheter held stationary in the coronary artery. The catheter tip design includes a rapid-exchange guidewire lumen (approximately 20mm long) and a radio-opaque marker band 2mm from the distal tip. The imaging optics (focusing lens and side-firing mirror) are contained in a radio-opaque protective housing, which makes up the distal terminus of a contra-helically wound multifilar torque cable.
Safety – porcine studies
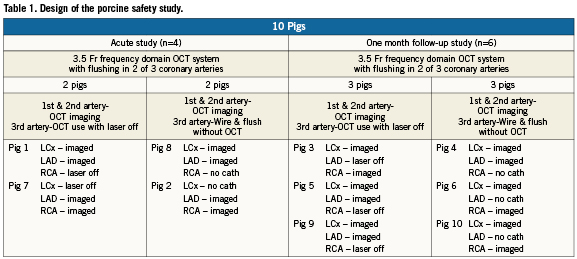
This study was conducted in compliance with the United States Food and Drug Administration (FDA) Good Laboratory Practices Regulations (21 CFR Part 58). Yorkshire pigs (40-50 kg, n=10) were studied. The pigs were divided into acute and chronic cohorts, and the primary endpoint for both cohorts was histology. The variables examined included the impact of power flushing with a 3.5Fr frequency domain OCT catheter with 50:50 saline: iodixanol (Visipaque; GE Healthcare, Princeton, NJ, USA), the same parameters but with the OCT laser turned off, and the same parameters in the absence of the OCT catheter (power flush with guidewire in place only). The acute study enrolled n=4 pigs, and evaluated n=12 coronary arteries. The chronic study enrolled n=6 pigs, and evaluated n=18 coronary arteries. A tabular summary of these protocols is given in Table 1. The acute porcine cohort was sacrificed immediately post-OCT imaging and the chronic cohort was sacrificed at one month post-OCT imaging.
Swine OCT imaging procedures
All swine were pre-treated with dual antiplatelet therapy (aspirin 325mg and plavix 75mg) for three days prior to the procedure. The pigs were anesthetised using telazol (4-6mg/kg I.M.) and following 2% isoflurane inhalation were secured in the dorsal position. Using aseptic technique, a vascular introducer sheath was placed in the right or left femoral artery by percutaneous artery puncture or surgical cutdown. Heparin 200 units/kg was administered via the introducer sheath or ear vein cannula; activated clotting time (ACT) was measured. If the ACT was >300 seconds the procedure continued; if not, an additional 50 units/kg of heparin was administered. Every 20-30 minutes, a repeat ACT was performed and additional heparin was administered accordingly. Pigs also received IV bolus 150mg of amiodarone to minimise the occurrence of ventricular fibrillation. A 6Fr guide catheter (JR4 for the right and hockey stick for the left coronary arteries) was advanced to the ascending aorta. The guide catheter engaged the ostium of the coronary arteries. Nitroglycerine 200µg was injected intracoronary. The 3.5Fr OCT catheter system followed the guidewire into the vessel and images were obtained as the OCT catheter was held stationary. While imaging, a 50:50 mixture of saline and Visipaque 350 (GE Healthcare, Princeton, NJ, USA) was power injected (Medrad, Pittsburgh, PA, USA) into the OCT imaged coronary arteries at a rate of 4ml/sec for six seconds (24ml per flush) to clear the blood in order to avoid RBC scatter of the OCT light. At the completion of imaging, the OCT catheter was removed from the swine. A final angiogram of the coronaries was performed; presence of vessel dissection, perforation or rupture, abrupt vessel closure and embolisation was documented.
The animals in the acute group were sacrificed and the hearts were removed for histopathology. For the 30-day follow-up group, the introducer was removed. Haemostasis was achieved by hand compression in the case of percutaneous access, or the femoral artery was ligated and the surgical wound repaired if a cutdown was performed. Pigs were recovered from anaesthesia, monitored to sternal recumbency, and returned to routine care. At termination, 30-day pigs were anesthetised as above; heparin 200 units/kg was injected and animals were terminated by intravenous injection of KCl while still under deep anaesthesia with inhalant isoflurane. Hearts were harvested and prepared for histopathology.
Histology endpoint
Hearts were excised and perfusion fixed at 100mmHg pressure via the aortic root, with 5% formalin/1.25% glutaraldehyde for 10 min, then immersed overnight in 10% formalin until further processing. Instrumented coronary artery segments were identified by anatomic landmarks and excised with 3-5mm thickness of adjacent, adherent perivascular tissue and myocardium. Transverse pieces 3mm thick were cut and placed in tissue processing cassettes; a minimum of five pieces within the treated coronary segment were sampled. They were dehydrated through graded ethanol, exchanged in xylene, and embedded in paraffin. Sections 5µm thick were cut on a rotary microtome and collected onto glass slides; adjacent sections were stained with haematoxylin-eosin and Verhoeff-Masson elastic-trichrome. Every slide was reviewed by an experienced pathologist.
Efficacy – porcine studies
It was unclear whether hand versus power injection, saline versus high viscosity solutions, and which rate of power injection will generate optimal OCT visualisation of the coronary arterial wall. Thus, Yorkshire pigs (40-50 kg, n=3) were enrolled in a randomised flushing study. We compared 100% saline to 50:50 solutions of saline and Visipaque (GE Healthcare, Princeton, NJ, USA); power injection rates of 4 and 6ml/sec; and hand versus power injection in both the proximal and middle portion of all three coronary arteries (n=9 in vivo coronary arteries). This research was approved by Institutional Animal Care and Use Committee at University of Texas Health Science Centre at San Antonio, TX, USA.
OCT imaging protocol
The imaging protocol was identical to that given above, with the following exception: the 3.5Fr Volcano frequency domain OCT catheter was advanced over the guidewire into either the middle or proximal coronary artery. The proximal OCT catheter position was defined as 10 mm distal to the left main endpoint, or right coronary ostium. Middle was defined as 40mm distal to the proximal position. The guide catheter engaged the left main at a depth halfway between the aorta and LAD/LCx bifurcation. In all three pigs studied, a second guidewire was passed into the non-imaged coronary artery to maintain a stable left main guiding catheter position. In that fashion, selective engagement of the left anterior descending or left circumflex coronary arteries was avoided. All OCT image acquisitions started two seconds before the flush was initiated, followed by a five second flush and subsequent three second OCT acquisition following termination of the flush (total 10 second OCT acquisition). The OCT catheter remained stationary and no pullback was performed during flushing. Solutions (100% saline, and 50:50 of saline and Visipaque) were either power injected at 4 cc/sec×5 sec and 6 cc/sec×5 sec injection, or hand injected by the same individual (20 ml injected over an average of 5 sec). The rise time for the power injector was 1.2 sec and the mean pressure was 122±41 psi.
Quantitative analysis of OCT images
For each image acquisition, 200 frames/10 seconds were obtained. Forty of these 200 frames were analysed. Each frame was analysed for the degrees of clearly visualised vessel wall provided by the flushing, and the area in degrees not visualised due to inadequate blood clearance was also determined. Guidewire artefacts projected onto the vessel wall and other non-analysable artefacts were excluded from this quantitative analysis. The guidewire occupied 17±5% of the vessel wall. The final measure of visualisation of the coronary wall was calculated as follows ×100%:

One hundred percent implies the entire vessel wall was visualised, and 0% implies none of the vessel wall was visualised. Visualisation of greater than 80% of the vessel wall was consistent with clinically interpretable images. All data were reviewed by three readers who were blinded to the design of flushing study, and their analyses averaged.
Statistics for all studies
In the randomised porcine flushing studies, the proportion of the circumference of the vessel visualised was measured every 0.25 seconds for 10 seconds in all coronary arteries, two locations (proximal/middle), three velocities (4 ml/sec, 6 ml/sec, hand injection), and two solutions (saline, 50:50 saline:Visipaque) in three pigs. The area under the curve (AUC) was computed for each combination. The significance of variance in the mean AUC with vessel, location, solution, and velocity was assessed with repeated measures linear models with a compound symmetric auto-covariance assumption. All statistical testing was two-sided with a significance level of 5%. SAS Version 9.2.3 for Windows (SAS Institute, Cary, NC, USA) was used.
Results
Safety – porcine studies
Final angiograms of the coronaries did not reveal the presence of vessel dissection, perforation or rupture, abrupt vessel closure, or embolisation in any of animals enrolled in this study.
Acute group
The acute changes to the vessel wall as defined by histology in the OCT group and laser-off group were minimal. These effects included minor focal endothelial desquamation associated with minimal platelet adhesion, mild oedematous changes in the inner layers of the media, and minor structural changes of the internal elastic lamina. The structures of the outer layers of the tunica media, as well as the tunica adventitia, were normal. There was only minimal inflammatory cell infiltrate into the vessel wall. More significant evidence of injury was absent including no instances of internal elastic lamina rupture, medial dissection injury, arterial wall perforation, or haemorrhage in any section examined.
In the flush-only group, the only effect of power injection alone on the vessel wall included minor focal endothelial sloughing and minimal focal or multifocal oedema in the innermost layers of the tunica media. Occasionally, nuclei of smooth muscle cells could also be seen to have a somewhat contracted appearance. For the vast majority of samples analysed, particularly in regions of these arteries located distally, were usually entirely normal in appearance. Typical examples of these findings are demonstrated in Figure1.
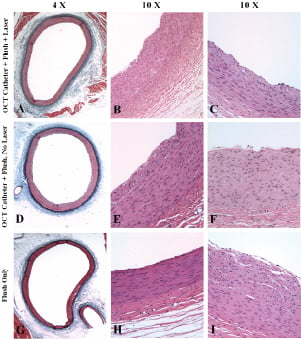
Figure 1. Hematoxylin-eosin and Verhoeff-Masson elastic-trichrome staining of coronary vessels in the acute group. A-C (OCT group) and D-F (laser off group): predominantly normal morphology; multifocal endothelial desquamation; subendothelial oedematous changes and minor oedema in innermost media. G-I (flush-only group): predominantly normal morphology; minor endothelial loss proximally; subendothelial oedematous changes; and minor oedema in innermost media.
One-month follow-up group
Histological examination of the OCT group and laser-off group revealed that chronic changes to the vessel wall were minimal. Only occasional and mild focal medial thickening that was spatially associated with very attenuated and focal fibrocellular neointima formation was identified. There were rare inflammatory cell infiltrates in any specimen. Complete recovery of the vascular endothelium was observed in every section of every vessel. There were no instances of internal elastic lamina rupture, medial dissection injury, arterial wall perforation, or haemorrhage in any sample.
Arteries harvested in the flush-only group appeared normal with only the most minimal of medial changes and rare neointima formation in the most proximal regions of the vessels. Typical examples of these findings are demonstrated in Figure 2.
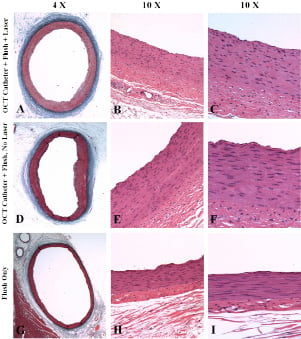
Figure 2. Hematoxylin-eosin and Verhoeff-Masson elastic-trichrome staining of coronary vessels in the one-month follow-up group.
A-C (OCT group) and D-F (laser off group): minimal focal media thickening; rare focal mild superficial medial fibrosis; minimal, focal, thin eccentric fibrocellular neointima formation; complete re-endothelialisation; virtually normal arterial wall morphology.
G-I (flush-only group): normal morphology and no apparent chronic effects.
Efficacy – porcine studies
Figure 3A shows the success of vessel wall clearance of 50:50 saline:Visipaque mixture versus saline, for all conditions (pooling all three coronaries; hand plus power injections; and proximal and middle positions). As is evident, there was improved vessel wall visualisation with 50:50 saline:Visipaque compared to saline alone (50:50 AUC [mean=22.32 (SD=5.87)], Saline alone AUC 15.74 [7.34]; p=0.04). Figure 3B is a typical example of 50:50 saline:Visipaque mixture versus saline raw OCT data.
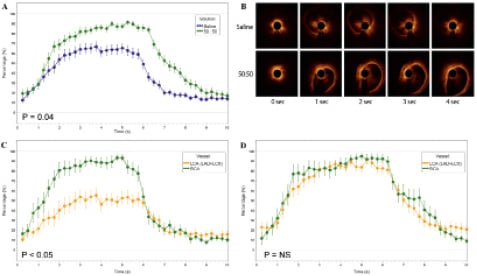
Figure 3. A) Demonstration that 50:50 saline:Visipaque improved arterial wall visualisation compared to saline alone for all conditions (p=0.04); B) Time sequence of OCT images comparing saline with 50:50 saline: Visipaque. As is evident, the 50:50 saline:Visipaque panels demonstrate earlier, longer, and more complete clearance of RBCs compared to saline (data previously published16); C)Demonstration that when injecting saline alone, improved vessel wall visualisation is obtained in the RCA compared to the LCA (p<0.05 during injection); D) Demonstration that the addition of Visipaque to saline (50:50) improves arterial wall visualisation to above 80% for the LCA, similar to the RCA (p=NS).
To determine the impact of half the injectate being lost during left main injection, the LAD and LCx were compared to the RCA for saline injection only (LAD and LCx AUC 11.90 [7.49], RCA AUC 19.59 [4.85]; p=0.60 Figure 3C). Improved vessel wall visualisation was demonstrated for the RCA compared to the LAD and LCx during flushing. When the same analysis was performed using 50:50 saline:Visipaque as the injectate, wall visualisation was not significantly different between the RCA compared to the LAD and LCx (LAD and LCx AUC 21.90 [5.33], RCA AUC 22.74 [6.50] p=0.27, Figure 3D).
Figure 4A compares two modes of power injection versus hand injection, for all conditions (pooling all three coronaries; saline and 50:50 saline:Visipaque; and proximal and middle positions). All three injection techniques examined were not significantly different (velocity4 AUC 17.33 [5.93], velocity6 AUC 19.55 [5.69], hand injection AUC 20.24 [9.80] ; 4 vs. 6: p=0.16; 4 vs. hand: p=0.13; 6 vs. hand: p=0.80). Similarly, no significant differences between techniques were found in time periods 0s~2s and 7s~8s (velocity4 AUC 1.85 [1.55], velocity6 AUC 2.88 [1.94], hand injection AUC 4.45 [2.88]; 4 vs. 6: p=0.11; 4 vs. hand: p=0.22; 6 vs. hand: p=0.34).
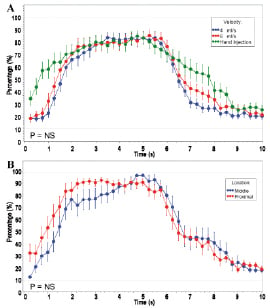
Figure 4. A) Randomised efficacy studies in n=9 porcine coronary arteries examining power injection versus hand injection on the percentage of the arterial wall visible. Hand injection, 4 ml/sec, and 6ml/sec all produced comparable and greater than 80% vessel wall visualisation; B)Randomised efficacy studies in n=9 porcine coronary arteries examining proximal and middle stationary OCT catheter positions from the guiding catheter tip, on the percentage of the arterial wall visible. Both positions provided comparable and greater than 80% vessel wall visualisation.
Figure 4 B compares the fixed proximal and middle OCT catheter positions in all the coronary arteries, for all conditions (saline and 50:50; and hand and power injections). No improved vessel wall visualisation was obtained in the proximal position compared to the middle position (proximal AUC 19.31 [6.11], middle AUC 18.76 [8.55]; p=0.76). When half the injectate was lost during left main injection, the same result was found (figure not shown).
To be certain that these results were not impacted by differences in the cross-sectional areas of the coronary arteries, these diameters were measured with OCT. Pairwise comparisons of the three vessel types with regard to vessel size were not significant (LAD vs. LCX [p=0.50], LAD vs. RCA [p=0.75], LCX vs. RCA [p=0.50]).
Discussion
To develop safe and efficacious methods for frequency domain imaging, we demonstrated the following: (a) that frequency domain imaging is safe in the porcine model using histology as the endpoint; (b) the addition of a viscous contrast (Iodixonal) to saline is superior for vessel wall clearance compared to saline alone; (c) hand injection, 4 ml/sec, and 6 ml/sec power injection all provided similar vessel wall clearance; and (d) the anticipated loss of vessel wall resolution with left main injection (due to half the injectate in the non-imaged vessel) was not evident in the proximal versus middle catheter positions.
High viscosity agents, which have more efficient ‘transfer of displacement energy’ to remove RBCs and delay RBCs’ reappearance in the imaging field, have been reported to achieve excellent image quality by others9,15,17. Since Iodixonal (Visipaque) is the most viscous of contrast agents18, it was examined in our study. We confirm that the 50:50 saline:Visipaque mixture has a significantly better image quality compared to 100% saline for all conditions (Figure3A and 3B). The 50:50 saline:Visipaque flushing solution allowed the left main injection to clear RBCs similar to the RCA injections (Figure 3C and 3D), while this was not true when flushing with saline alone. 100% Visipaque, which has an even higher viscosity, was not utilised in our porcine efficacy studies, since it was too difficult to inject by hand through the 6 Fr guiding catheter containing the 3.5 Fr frequency domain OCT catheter.
Successful vessel wall visualisation was defined as greater than 80%. The ability to obtain similar 80-90% clearance of the arterial wall with either hand or power injection provides clinicians the option of utilising either technique. However, hand injection and 4ml/sec techniques both provided the same volume (20ml) over the same time duration (5 sec). When increasing the power injection to 30 ml over five seconds (6 ml/sec), the same acceptable clearance was observed. Thus, 6 ml/sec injection rates are probably not necessary.
The rationale for investigating the effect of flushing coronary arteries with wires without the OCT catheter present, or with the OCT catheter present but the laser off, is given below. There are currently no interventional cardiology procedures that combine power injection into a coronary artery, pullback of an imaging device, and activation of an OCT laser to image the arterial wall all at the same time. Compounding this issue is that the frequency domain OCT catheters are of a much greater diameter than the time domain OCT catheters, adding to the resistance of the injection to clear blood from the imaging field. There was concern during study initiation that there would be damage identified at autopsy by histology in the porcine coronary arteries. Thus, the study was designed to dissect out all the variables –the laser, the large diameter of the OCT catheter, and the power injection. For this reason, two experimental groups were added to the study –laser off and absence of the OCT catheter in the coronary artery at the time of flushing. There was surprise at the absence of any coronary artery damage identified with histology in any of the three groups examined, but this outcome was not anticipated during study design.
One limitation of these studies was that the OCT catheter was stationary and not pulled back, to eliminate the pullback as a source of variability. The ability to obtain greater than 80% visualisation of the arterial wall regardless of whether the stationary OCT catheter was placed in a proximal or middle coronary artery position was reassuring. Despite half the injectate being diverted into the non-imaged coronary artery for the left main guiding catheter position, excellent clearance of RBCs was still possible. Care was taken to assure that the tip of the 6 Fr guiding catheter was placed in the middle of the left main. Since the porcine model has a short left main, a second guidewire was passed into the left circumflex (LCx) to hold the guiding catheter tip in a stable position, although not recommended during clinical imaging in patients. Whether adequate vessel wall clearance is possible in the distal LAD and/or LCx was not addressed in the current study.
Another limitation of the flushing study is that a randomised scheme was not utilised. As a result, larger numbers of vessels were flushed as controls (traditional OCT technique), and there were lesser experimental studies (OCT catheter present in coronary artery but laser turned off, and wire and flush used in coronary artery without the OCT catheter). Thus, the results of this study are primarily descriptive.
In conclusion, frequency domain OCT imaging is safe without histologic evidence of trauma to the vessel wall, ideally performed with 50:50 mixtures of saline:Visipaque to clear the coronary of RBCs, and injected by either hand or a power injector, including from the left main position.
Conclusions
The Volcano 3.5 Fr frequency domain OCT catheter system was investigated for safety in n=10 porcine studies and efficacy in n=9 in vivo porcine coronary arteries. We found: (a) frequency domain imaging is safe using histology endpoint; (b) Iodixonal and saline mixture is superior for vessel wall clearance compared to saline alone; (c) hand injection, 4 ml/sec, and 6ml/sec power injection all provided similar vessel clearance; (d) the anticipated loss of vessel wall visualisation with left main injection was not evident in the proximal versus middle coronary artery OCT catheter position. Frequency domain OCT is safe and efficacious.
Acknowledgement
This study could not have been completed without the assistance of Jennifer Rumpf, Christopher Mittal, Pauline Nguyen, Isabel Lopez, Yasien Osama Eltigani, and Ernest Thomas.
Sources of funding
This study was supported in part by Volcano Corporation, San Diego, CA, USA and a VA Merit Grant (MDF)
Conflict of interest statement
The authors have no conflict of interest to declare.