Abstract
Aims: This study sought to assess whether renal sympathetic denervation (RSD) could suppress ventricular substrate remodelling and attenuate heart failure (HF) progression.
Methods and results: Nineteen dogs were randomised into three groups – seven sham-operated controls, six with right ventricular pacing to induce HF, and six with RSD followed eight weeks later by pacing induction of HF. Haemodynamic variables were monitored at baseline and after HF. Levels of ventricular interstitial fibrosis, BNP, Ang II, aldosterone and TGF-β were measured. All the dogs in the HF and HF﹢RSD groups showed increased left and right ventricular diastolic dimensions, but the dogs in the HF﹢RSD group had a higher left ventricular ejection fraction (LVEF) than the HF dogs (0.42±0.05 vs. 0.35±0.04, p<0.01). Compared with the dogs with HF alone, the HF+RSD dogs had lower left ventricular end-diastolic pressure (LVEDP) (3.3±1.6 vs. 25±3.7 mmHg, p<0.01) and less fibrous tissue. The levels of BNP, Ang II, aldosterone and TGF-β expression in ventricular tissue were higher in the HF dogs than in the sham-operated and HF﹢RSD dogs.
Conclusions: RSD suppressed ventricular substrate remodelling induced by long-term rapid ventricular pacing.
Introduction
Studies have demonstrated that the renin-angiotensin-aldosterone system (RAAS) plays a key pathophysiologic role in the progression of ventricular dysfunction and heart failure (HF)1,2. Activation of the RAAS leads to increased levels of angiotensin II (Ang II) and plasma aldosterone and promotes development of cardiac remodelling, oxidative process, and cardiac fibrosis3,4. The beneficial effects of angiotensin-converting enzyme (ACE) inhibition on morbidity and mortality in all stages of HF are well documented and may possibly extend to disease prevention5,6.
It is well known that the congestive HF state is characterised by elevated sympathetic tone and depressed cardiac vagal tone. Studies have shown that dogs with congestive HF exhibit enhanced renal sympathetic nerve activity (RSNA) responses to electrical stimulation of the cardiac sympathetic afferent nerves7,8. Administration of the AT1 receptor antagonist losartan attenuates the RSNA response to stimulation in congestive HF9. Our previous study showed that renal sympathetic denervation (RSD) inhibits RAAS activity10. Whether RSD can suppress ventricular substrate remodelling and attenuate HF progression is unknown. In the present study, we examined the effect of RSD on HF progression and ventricular substrate remodelling in a canine high-rate pacing model.
Methods
Animal handling was performed according to the current guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised 1996). The study protocol was approved by the Ethics Committee of Wuhan University. Animal handling was carried out according to the Wuhan Directive for Animal Research.
EXPERIMENTAL MODELS
Nineteen adult mongrel dogs of both sexes and unknown age, weighing 19.5±2.6 kg, were divided into three groups: sham-operated group (n=7), HF group (n=6) and HF﹢RSD group (n=6). An intramuscular injection of 25 mg/kg of ketamine sulphate was administered to all the dogs. The dogs were then premedicated with pentobarbital sodium (30 mg/kg IV; additional doses of 4 mg/kg were administered when required throughout the experiment), intubated, and ventilated using a respirator with room air supplemented with oxygen (MAO01746; Harvard Apparatus, Holliston, MA, USA). Continuous ECG monitoring was carried out using leads I, II and III.
In the sham-operated group, a pacemaker (made at Shanghai Fudan University, China) was implanted in a subcutaneous pocket and attached to a pacing lead (Capture Sense; Medtronic Inc., Minneapolis, MN, USA) in the right ventricular apex under fluoroscopic visualisation via the right external jugular vein. When the surgery was completed, the dogs were given antibiotics and then allowed to recover for three weeks without pacing. In the HF group, after pacemaker implantation, the dogs were given antibiotics and allowed to recover for three days. They subsequently underwent rapid ventricular pacing at 240 beats per minute (bpm) for three weeks.
The dogs in the HF﹢RSD group underwent renal artery ablation (RAA) before rapid ventricular pacing. Briefly, a 6 Fr ablation catheter was introduced (Biosense Webster, Inc., Diamond Bar, CA, USA) into each renal artery via the femoral artery10. We applied discrete radiofrequency ablations of 6 watts or less for up to 80 sec each within each renal artery. During ablation, the catheter was advanced and retracted three times from distal to proximal in the renal arterial lumen. Antibiotics were administered for three days after ablation and the animals were allowed to recover for eight weeks. After that, a pacemaker was implanted, and the dogs underwent rapid ventricular pacing at 240 beats per minute (bpm) for three weeks.
Femoral artery blood pressure was measured in all the animals at baseline and after pacing.
ECHOCARDIOGRAPHY AND HAEMODYNAMIC EVALUATION
Transthoracic 2D and Doppler echocardiography was performed in all the animals using Vivid 7 (GE Healthcare, Pittsburgh, PA, USA) before pacemaker implantation and after three weeks. Standard 2D short- and long-parasternal views, as well as 4-chamber, 2-chamber, and 3-chamber apical views were obtained. The left ventricular diastolic dimensions (LVDD), right ventricular diastolic dimensions (RVDD) and left ventricular ejection fraction (LVEF) were measured by using a Simpson’s biplane equation. All volumes were measured in triplicate, and averages are reported. The images and the parameters were reviewed by an independent echocardiography expert.
Haemostatic sheaths were inserted into the right and left femoral arteries in all the dogs after three weeks of pacing. Femoral artery systolic, diastolic, and mean pressures as well as right and left ventricular systolic and diastolic pressures were measured. A multipolar electrode catheter was introduced into the femoral vein and placed in the right ventricular apex. After the pacemaker had been closed for 30 min, right ventricular pacing was performed via a multipolar electrode at 200 bpm. Haemodynamic variables were monitored continuously until the values were stable (usually within five minutes).
ELISA AND WESTERN BLOT STUDIES
Four millilitres of venous blood were collected in EDTA vacutainers and centrifuged at 2,310 g for 10 min at 4°C (Avanti J-E; Beckman Coulter, Brea, CA, USA) before and after pacing for three weeks. The serum was separated into microtubes and stored at –80°C until analysis. Plasma BNP (Canis BNP Elisa Kit, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), Ang II (Canis Ang II Elisa Kit, Nanjing Jiancheng Bioengineering Institute), and aldosterone (Canis Aldosterone Elisa Kit, Nanjing Jiancheng Bioengineering Institute) concentrations were measured by ELISA. Tissue samples were obtained from the left and right ventricular anterior walls in all the animals after haemodynamic measurement. Levels of BNP, Ang II and aldosterone in the left ventricular anterior wall were measured by ELISA.
Expression of TGF-β in the left and right ventricular free walls was measured by western blot. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween 20 (TBST) for one hour and incubated overnight at 4°C with the primary antibodies (monoclonal rabbit anti-TGF-β1 antibodies; Abcam, Cambridge, UK) used at 1:1,000; goat anti-Smad2 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) used at 1:500; rabbit anti-actin antibody (Santa Cruz Biotechnology, Inc.) used at 1:1,000. They were then washed in TBST three times, incubated with the secondary antibody for one hour at 37°C, and imaged using the Immun-Star horseradish peroxidase substrate. The relative expression levels of the protein were determined using image analyser software (AlphaEase FC; Alpha Innotech, San Leandro, CA, USA).
HISTOLOGICAL EVALUATION
Right and left sections of the ventricular anterior wall were dissected from the heart and immediately stored at -80°C. Masson’s trichrome staining was used to identify increased concentration of interstitial fibrosis. Connective tissue was differentiated on the basis of its colour, and the level was expressed as a percentage of the reference tissue area (Ti-S; Nikon, Tokyo, Japan). Blood vessels and perivascular interstitial cells were excluded from the connective tissue quantification. The interstitial collagen volume fraction in the ventricle was determined by quantitative morphometry with an image analyser (IPP 6.0; Media Cybernetics, GA, USA).
STATISTICAL ANALYSIS
The values are shown as the mean+SD. In addition to comparing the results of the LVDD and RVDD measurements during the post-study period for the HF and HF+RSD groups using a paired t-test, two-sample independent Student’s t-tests were used to compare the means of two groups. ANOVAs with Newman-Keuls tests were used to compare the means of continuous variables among multiple groups and, in the case of a significant difference, further analysis was undertaken with a Tukey-Kramer test. All the statistical tests were two-sided, and a probability value <0.05 was required for statistical significance.
Results
During three weeks of observation, there was no change in the weight of the sham-operated dogs (19.7±2.4 to 20.0±2.6 kg, p=NS). There was also no change in the HF and HF﹢RSD dogs who were paced for three weeks (19.4±2.6 to 21.1±3.8 kg and 19.6±2.6 to 21.4±3.5 kg, respectively, both p=NS). No differences were found in the weights between any of the groups.
In the HF﹢RSD group, the femoral artery systolic pressures were 164±13 mmHg at baseline, 133±11 mmHg eight weeks after RAA and 118±10 mmHg after pacing for three weeks. In the HF group, the femoral artery systolic pressures were 159±14 mmHg at baseline and 122±11 mmHg after pacing for three weeks. There was a significant decrease in the femoral artery systolic and diastolic pressures after pacing in both groups (Table 1). There was a slight but insignificant decrease in systolic pressure after three weeks in the sham-operated group (158±16 vs. 151±17 mmHg, p>0.05).
HAEMODYNAMIC EFFECTS OF PACING
The haemodynamic results obtained are shown in Figure 1 and Table 1. After the pacemaker had been closed for 30 min, haemodynamic measurements were performed. In the sham-operated dogs, the right ventricular end-systolic pressure (RVESP), right ventricular end-diastolic pressure (RVEDP), left ventricular end-systolic pressure (LVESP) and left ventricular end-diastolic pressure (LVEDP) were 30±5.4 mmHg, 4.1±1.2 mmHg, 163±17 mmHg, 2.5±1.3 mmHg, respectively. By contrast, the dogs which were paced at 200 bpm exhibited sharply reduced LVESP (75±14 mmHg) and increased LVEDP (9±2.4 mmHg), which increased gradually to a stable level of 158±21 mmHg after 20±3 sec.
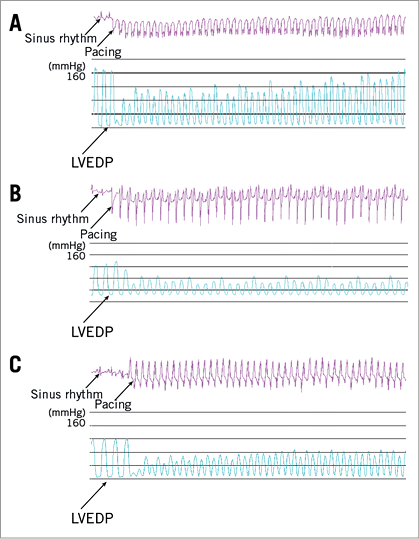
Figure 1. Changes of left ventricular pressure in the three groups during rapid-burst right ventricular pacing. A) Changes in the LVESP in sham-operated dogs during rapid-burst right ventricular pacing. The results showed that the LVESP sharply decreased and increased gradually during rapid-burst right ventricular pacing. B) Changes in the LVESP in HF dogs during rapid-burst right ventricular pacing. The results showed that there was a sharply reduced LVESP but had no significant recovery. The HF dogs had higher LVEDP than that in sham-operated dogs. C) Changes in the LVESP in HF+RSD dogs during rapid-burst right ventricular pacing. The results showed that there was also a sharply reduced LVESP and had no significant recovery. However, the HF+RSD dogs had lower LVEDP than the HF dogs.
In the HF group, the dogs exhibited reductions in LVESP and higher LVEDP as compared with baseline (121±12 vs. 167±21 mmHg and 25±3.7 vs. 2.5±1.3 mmHg, respectively, both p<0.01). During rapid-burst pacing at 200 bpm, a sharply reduced LVESP was also exhibited. However, the LVEDP did not change significantly and the LVESP did not recover significantly. The dogs in the HF﹢RSD group also exhibited reductions in LVESP as compared with baseline (120±10 vs. 163±17 mmHg, p<0.01). Interestingly, the LVEDP in the HF﹢RSD group was lower than that in the HF dogs (3.3±1.6 vs. 25±3.7 mmHg, p<0.01), but there was no significant difference between the sham-operated group and the HF﹢RSD group (3.3±1.6 vs. 2.5±1.3 mmHg, p>0.05). During rapid-burst pacing, a sharply reduced LVESP was also observed, and the LVESP did not recover significantly.
ECHOCARDIOGRAPHY
At three weeks, there was no significant change in the sham-operated dogs either in wall thickness or in the left or right ventricular dimensions and volumes. By contrast, in the HF and HF+RSD dogs there was a marked reduction in the percentage of thickening in the left and right ventricular anterior walls. In addition, significantly increased LVDD and RVDD (HF dogs: LVDD, 27+2.4 vs. 37+2.8 mm, p<0.01; RVDD, 11+1.5 vs. 14+1.7 mm, p=0.03; HF+RSD dogs: LVDD, 30+2.5 vs. 36+2.8 mm, p=0.03; RVDD, 12+1.1 vs. 14+1.2 mm, p=0.04) and reduced left ventricular ejection fraction (LVEF) were noted, but the dogs in the HF+RSD group had higher LVEF than the HF dogs (0.42±0.05 vs. 0.35±0.04, p<0.01) (Table 2).
BIOCHEMICAL MEASURES AND WESTERN BLOT STUDIES
Compared with baseline, the plasma concentrations of Ang II and aldosterone were markedly decreased eight weeks after RAA in the HF+RSD group (Ang II: 154.8±26.2 vs. 82.4±17.3 pg/ml, p<0.01; aldosterone: 238.6±31.7 vs. 143.6±41.3 pg/ml, p<0.01). There was an increase in BNP, Ang II and aldosterone concentrations after three weeks of pacing as compared with baseline in the HF group (BNP: 61.4±9.5 vs. 174.7±18.5 pg/ml, p<0.01; Ang II: 143.9±20.6 vs. 275.1±34.3 pg/ml, p<0.01; aldosterone: 226.2±27.8 vs. 415.7±47.1 pg/ml, p<0.01). Importantly, when compared with those in HF dogs, the plasma BNP, Ang II and aldosterone concentrations were lower in the HF+RSD group (BNP: 93.7±11.8 vs. 174.7±18.5 pg/ml, p<0.01; Ang II: 177.2±25.1 vs. 275.1±34.3 pg/ml, p<0.01; aldosterone: 303.5±31.2 vs. 415.7±47.1 pg/ml, p<0.01).
The levels of BNP, Ang II and aldosterone in the left ventricular anterior wall tissue samples in the HF group were higher than those in the sham-operated group (BNP: 2.13±0.22 vs. 0.54±0.08 pg/mg, p<0.01; Ang II: 8.3±0.5 vs. 5.7±0.6, pg/mg, p<0.01; aldosterone: 194±19.3 vs. 75±12.4 pg/mg, p<0.01). When compared with those in the HF group, levels of BNP, Ang II and aldosterone in the left ventricular anterior wall tissues samples were lower in the HF+RSD group (BNP: 2.13±0.22 vs. 1.08±0.11 pg/mg, p<0.01; Ang II: 8.3±0.5 vs. 6.2±0.7 pg/mg, p<0.01; aldosterone: 194±19.3 vs. 115±16.2 pg/mg, p<0.01).
Figure 2 compares the western blots of the ventricles in the three groups. All the immunoblot band intensity measurements were normalised to the GAPDH band intensity of the loaded sample. The band densities were determined and they allowed for the relative quantification of the TGF-β levels. As shown in Figure 2A and Figure 2B, the expression of TGF-β in the right ventricle (RV) was higher in the HF group than in the sham-operated and HF+RSD groups (0.4 vs. 0.22 and 0.4 vs. 0.27, respectively, p<0.01). There was no significant difference in the LV between the HF+RSD group and the HF group (0.39 vs. 0.45, p=0.51).
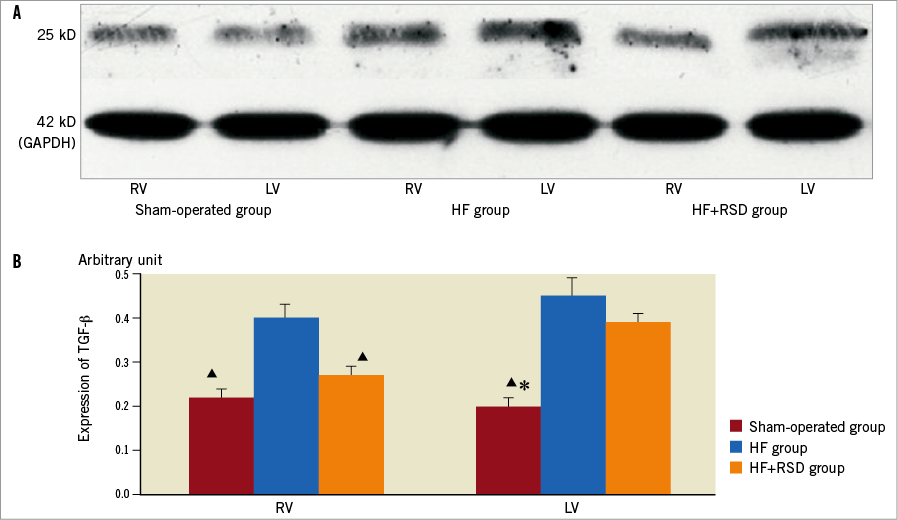
Figure 2. Western blot analysis of TGF-β levels in the protein sample extracts from the RV and LV. A) Examples of the western blot bands recognised by the TGF-β antibodies (25 kD). B) Mean band densities expressed as RV and LV ratios. The HF group over the sham-operated group and the HF+RSD group, ![]() p<0.01. The HF+RSD group over the sham-operated group, * p<0.01.
p<0.01. The HF+RSD group over the sham-operated group, * p<0.01.
HISTOLOGICAL CHANGES
Masson’s trichrome staining was used to evaluate the extent of fibrosis in the tissue sections. The right and left free ventricular walls in the three groups were composed of red myocardial tissue and blue collagen fibres (Figure 3). Left and right ventricular images from HF hearts revealed a large amount of fibrosis in the ventricle (LV: 24.1±4.8%; RV: 17.2±5.2%), whereas the sham-operated dogs showed minimal fibrous tissue (LV: 4.2±1.7%; RV: 3.6±1.2%). By contrast, the left and right ventricular sections from the HF+RSD animals had significantly less fibrosis than the HF dogs (LV: 8.5±1.9% vs. 24.1±4.8%, p<0.01; RV: 11.8±3.9% vs. 17.2±5.2%, p<0.01).
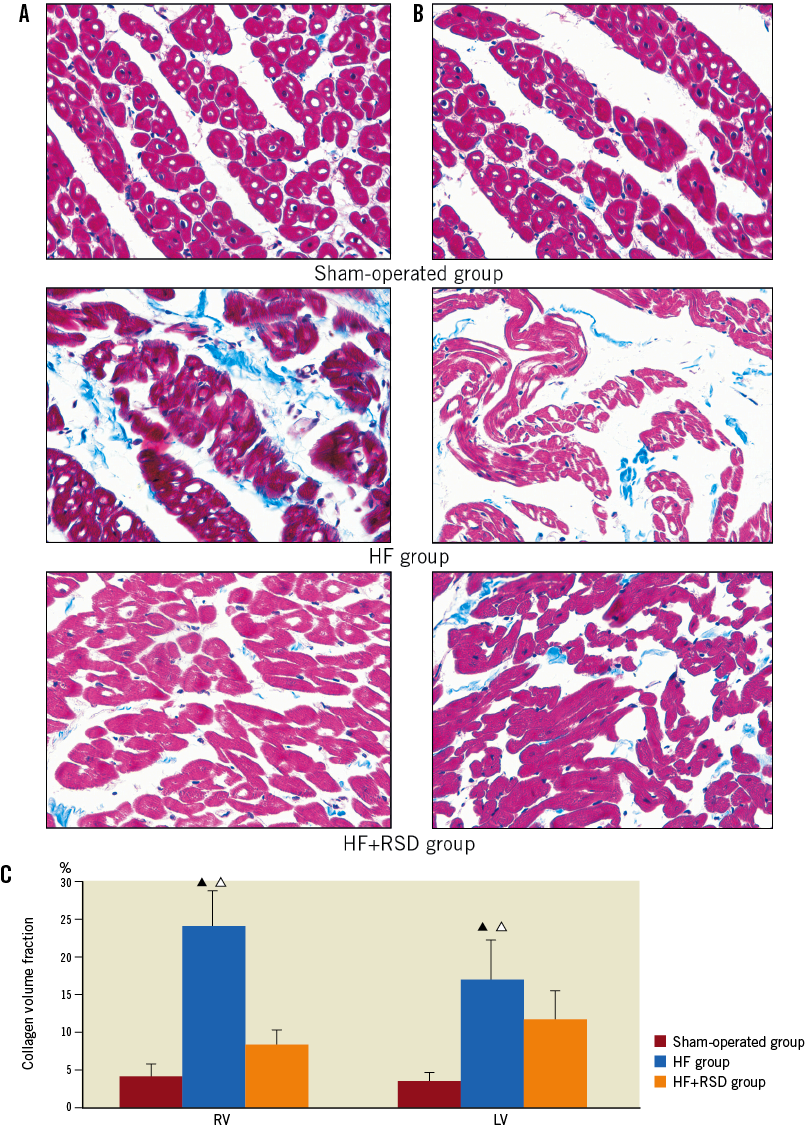
Figure 3. Histological changes in ventricular tissues. Red areas represent myocytes; blue areas represent collagen (original magnification 400). A) Examples of sham-operated, HF and HF+RSD left ventricle. B) Examples of sham-operated, HF and HF+RSD right ventricle. C) Summary of changes in the left and right ventricles. By contrast, the left and right ventricular sections in the sham-operated and HF+RSD dogs had less fibrosis (![]() p<0.01, vs. the sham-operated group;
p<0.01, vs. the sham-operated group;
p<0.05, vs. the HF+RSD group).
Discussion
MAJOR FINDINGS
Long-term RSD suppressed the increased levels of circulating hormones and inhibited ventricular substrate remodelling during rapid ventricular pacing. The effects might be associated with decreased activity of the RAAS after RSD.
RAAS, NEUROHUMORAL VARIABLES AND HF
Activity of the RAAS is increased in patients with HF, and its maladaptive mechanisms may lead to adverse effects such as cardiac remodelling and sympathetic activation. RAAS activity mutually influences the secretion of neurohumoral factors, such as renin, Ang II and aldosterone et al. Furthermore, plasma renin activity is used as an index of RAAS activity. Several studies have demonstrated that a prolonged overactivation of neurohormonal mechanisms contributes to drive structural and functional abnormalities of the cardiovascular system and results in a poor prognosis in patients with congestive HF. The activated RAAS contributes to myocyte hypertrophy, fibroblast hyperplasia and increased collagen deposition, whereas an inhibition of the RAAS mitigates left ventricular remodelling in the failing heart11-14. In several large-scale trials, suppression of the RAAS with various ACE inhibitors was proven to reduce the combined endpoints of mortality and morbidity and improve clinical signs and symptoms in patients with HF15,16.
RENAL SYMPATHETIC NERVE ACTIVITY AND HF
Previous studies have suggested that arterial baroreflex control of renal nerve activity is preserved in a high-output model of HF17. It has been shown that dogs with HF exhibit enhanced renal sympathetic nerve activity responses to electrical stimulation of the cardiac sympathetic afferent nerves. Chronic increases in circulating Ang II increased sympathetic nerve activity, which was related to arterial baroreflex resetting18. A study recently suggested that renal sympathetic nerve activity has a detrimental effect on renal blood flow and may be associated with alterations in the expression of Ang II receptors19. DiBona and Sawin showed that RSNA is significantly increased in rats with HF, and bilateral RSD has been shown in this model to normalise renal sodium handling20. Our previous study demonstrated that RSD inhibits the activity of RAAS10. Whether RSD has beneficial effects on HF is unknown.
Our results are in accordance with the previous investigations that have shown changes in neurohormonal factors during HF21-23. Interestingly, we found not only that rapid-pacing-induced HF could induce increased levels of Ang II, aldosterone and TGF-β in ventricular substrates, but also that these changes were suppressed during rapid-pacing-induced HF after RSD. As compared with the initial haemodynamic measurements, the HF dogs had increased LVEDP and lower LVEF. Surprisingly, in sharp contrast, the RSD dogs had lower LVEDP and higher LVEF than the HF dogs. These findings suggest that RSD attenuates the deterioration of myocardial function during rapid-pacing-induced HF. This study shows that the RAAS is involved in myocardial fibrosis in HF and that locally produced Ang II is associated with reactive interstitial fibrosis24,25. Blockade of the angiotensin II type 1 (AT1) receptor suppresses TGF-β upregulation26,27. Our results suggest that suppression of increases in levels of neurohormones and fibrosis is associated with mild deterioration of myocardial function after RSD.
CATHETER-BASED RENAL DENERVATION AS A NEW STRATEGY FOR HF MANAGEMENT
HF is a leading cause of hospitalisation and mortality. Despite advances in the control of cardiovascular diseases such as myocardial infarction, the incidence and prevalence of HF continue to increase28. The prognosis remains poor for many patients with HF, despite maximal medical treatment with ACE inhibitors, diuretics and digitalis. β-blockers are well established as a part of the standard therapy for patients with congestive HF: they have been shown to reduce mortality and to improve quality of life29,30. However, the beneficial effects of β-blockers are thought to be limited to certain drugs in the class. β-blockers with intrinsic sympathomimetic activity (xamoterol) and other agents, including bucindolol, have not demonstrated a survival benefit31,32. Although a combination of behavioural, pharmacological, device-based, and surgical treatment modalities has tremendously enhanced the survival and quality of life of patients with HF, successful management of this population depends on primary prevention and novel treatment strategies. In our study, RSD suppressed deterioration of ventricular function and inhibited ventricular substrate remodelling in a canine high-rate pacing model. RSD may provide a novel therapeutic approach for HF treatment.
Study limitations
The present study had several limitations. First, the time course over which ventricular substrate remodelling occurs was not studied. Thus, it is unknown whether the ventricular substrate remodelling begins shortly after the initiation of pacing or only after neurohormonal factors have had an effect. Second, because we did not perform stimulation of the renal sympathetic nerve, we could not determine the relative importance of renal sympathetic nerve activation and HF development. Third, the effectiveness and safety of catheter-based renal denervation have been demonstrated in patients. The renal arteries had no obvious stenoses and the plasma creatinine concentrations were not significantly different after RSD, but it is difficult to determine the presence of all potential side effects in animal studies. For example, our results showed that blood pressure decreased significantly after RSD. Whether a long-term decrease in blood pressure has potential side effects is not known. Clearly, the potential side effects associated with RSD will eventually need to be addressed in patients with mild hypertension or no hypertension. Lastly, HF that is provoked by ischaemia is more realistic than rapid pacing. Further studies should be designed to investigate the effect of RSD on HF that is provoked by ischaemia.
Conclusions
Catheter-based SD promoted cardiac function and suppressed ventricular substrate remodelling induced by long-term rapid ventricular pacing. The inhibition of RAAS activity may be a mechanism underlying these results.
| Impact on daily practice Chronic HF is a progressive syndrome that results in a poor quality of life for patients. When RAAS is chronically activated in HF, it has negative effects on the structure and function of the heart. We found that RSD suppressed deterioration of ventricular function and inhibited ventricular substrate remodelling in experimental HF. These findings may provide a novel therapeutic approach for HF treatment. In daily practice, we may introduce the method to prevent or treat HF. |
Funding
This study was supported by the National Science and Technology Pillar Program of China (2011BAI11B12), National Key Basic Research Development Program of China (The “973” Program, 2012CB518604) and the National Natural Science Foundation of China (81070144).
Conflict of interest statement
The authors have no conflicts of interest to declare.

