Abstract
Aims: We aimed to investigate whether sympathetic denervation of the heart and kidney had similar effects on ventricular effective refractory period (ERP) and action potential duration (APD) restitution properties in a canine model.
Methods and results: Twenty-four anaesthetised open-chest dogs (17-20 kg) were assigned to a sham operation group (n=8), a cardiac sympathetic denervation group (CSD, n=8) or a renal sympathetic denervation group (RSD, n=8). CSD was performed by ablating the caudal half of the LSG and T2-T4 thoracic sympathetic ganglia, while RSD was performed by ablating four sites on the adventitial surface of each renal artery. The ventricular electrophysiological properties were determined at four time points: baseline, 0 min, 30 min, and 60 min after interventions. The results showed that, when compared to the control group at the time point of 60 min after interventions, both CSD and RSD significantly reduced heart rate, prolonged the QT interval and ventricular ERP and APD, decreased the ERP dispersion and the slopes of APD restitution curves, and suppressed the APD alternans without affecting blood pressure and corrected QT interval. However, there were no significant differences in these parameters between CSD and RSD groups at the same time point.
Conclusions: This study showed that sympathetic denervation of the heart and kidney induced similar electrophysiological effects in ventricles.
Abbreviations
APD: action potential duration
BP: blood pressure
CSD: cardiac sympathetic denervation
ERP: effective refractory period
HR: heart rate
LSG: left stellate ganglion
LVM/RVM: the median area of left/right ventricular free wall
PVI: pulmonary vein isolation
QTc: QT interval corrected for HR
RSD: renal sympathetic denervation
RSN: renal sympathetic nerve
Smax: maximal slope
VA: ventricular arrhythmia
Introduction
Previous studies in humans1-4 and in animal models5,6 have shown that cardiac sympathetic denervation (CSD) by left stellate ganglion (LSG) block or resection is associated with a reduction in the incidence of ventricular arrhythmia (VA) and sudden cardiac death. Recently, several small sample studies7-10 have reported a new approach which reduced sympathetic activity by renal sympathetic denervation (RSD) to treat ventricular tachycardia storm. The results of these studies showed that both CSD and RSD displayed antiarrhythmic activity.
Although CSD has been shown as an effective therapy for human VA, it is performed by a left cervicothoracic surgery1-4 which may cause some undesirable side effects, such as unmanageable bleeding and Horner’s syndrome. In contrast, RSD, which can be performed via an intra-arterial approach7-14, would solve the problems encountered with CSD. Therefore, RSD may be an effective alternative to CSD for the treatment of malignant VA. In the present study, we aimed to investigate whether sympathetic denervation of kidney and heart induced similar effects on ventricular electrophysiology.
Methods
ANIMAL PREPARATION
All animal studies were reviewed and approved by the animal experimental administration of Wuhan University. The investigation conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication No. 85-23, revised 1996). Twenty-four adult mongrel dogs (17-20 kg) were anaesthetised with Na-pentobarbital (30 mg/kg) and ventilated with room air by a positive pressure respirator (MAO01746; Harvard Apparatus, Holliston, MA, USA). Additional maintenance doses of 2 mg/kg Na-pentobarbital were administered at the end of each hour. Heart rate (HR), blood pressure (BP), and standard electrocardiogram leads were continuously monitored. The QT interval corrected for HR (QTc) was calculated using Bazett’s formula. The chest was entered via a bilateral thoracotomy at the 5th intercostal space. Two multi-electrode catheters were sutured to left and right ventricular free walls to allow pacing and recording at six epicardial sites from the apex to the base (Figure 1A-Figure 1C). A custom-made Ag-AgCl catheter was used to record the monophasic action potentials from the median area of the left and right ventricular free walls (LVM and RVM).
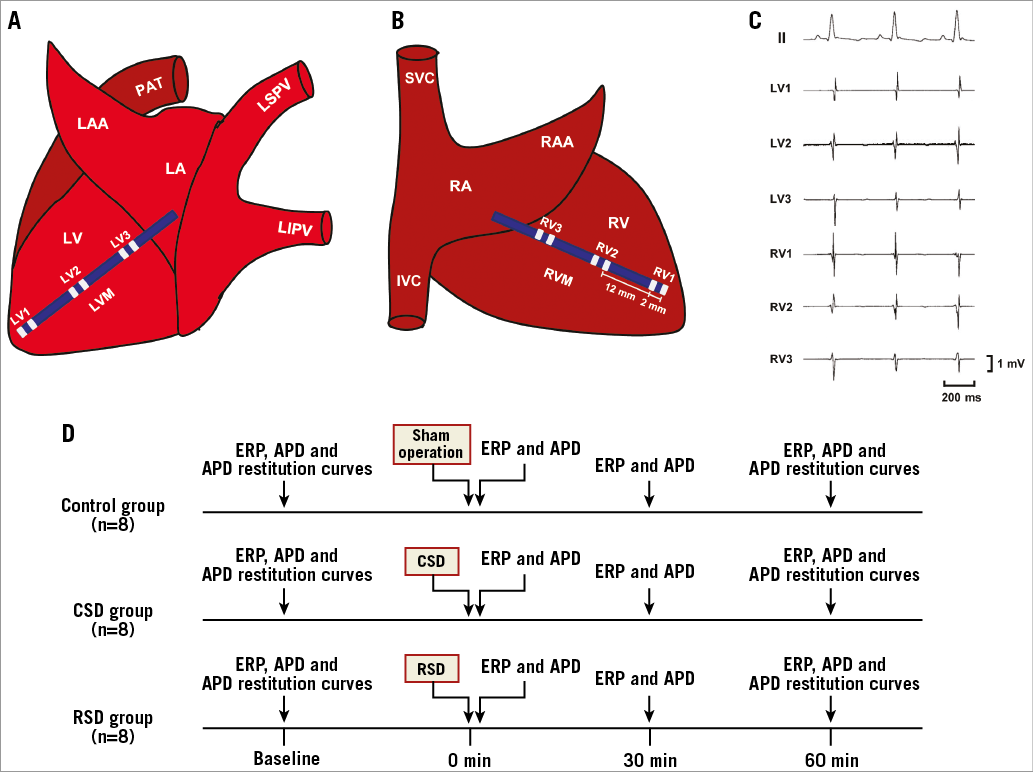
Figure 1. Schematic representation of catheter position at the left (A) and right (B) ventricular free walls, simultaneous surface and local cardiac electrocardiograms recording (C), and experimental design (D). APD: action potential duration; CSD: cardiac sympathetic denervation; ERP: effective refractory period; IVC: inferior vena cava; LAA: left atrial appendage; LA: left atrium; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; LV: left ventricle; LVM: the median area of LV; PAT: pulmonary artery trunk; RA: right atrium; RAA: right atrial appendage; RIPV: right inferior pulmonary vein; RSD: renal sympathetic denervation; RSPV: right superior pulmonary vein; RV: right ventricle; RVM: the median area of RV; SVC: superior vena cava
Sympathetic denervation
CSD
CSD was performed by ablation of LSG according to the previous studies2,15. After a left thoracotomy was performed, the caudal half of the LSG and T2-T4 thoracic sympathetic ganglia were identified by applying high-frequency stimulation (20 Hz, 2 ms pulse duration, 10 mA) with an irrigated large-tip (3.5 mm) electrode catheter (Biosense Webster Inc., Diamond Bar, CA, USA) and were ablated by delivering radiofrequency current (≤35 W, 60 s) to the sites showing blood pressure elevation during stimulation. Complete ablation was verified by elimination of blood pressure elevation when delivering electrical stimulation to the ablated area.
RSD
After a left or a right retroperitoneal flank incision was performed, high-frequency stimulation (20 Hz, 2 ms pulse duration, 15 mA, 60 s) was applied to identify the renal sympathetic nerves (RSN) under direct vision. Radiofrequency current (6-8 W, 60 s) was delivered to the sites showing blood pressure elevation during high-frequency stimulation. Ablation was considered complete when stimulation of each ablated site no longer induced any changes of blood pressure. Four sites in each renal artery were ablated to achieve bilateral RSD.
DETERMINATION OF VENTRICULAR ERP
Ventricular ERP was defined as the longest S1-S2 interval that failed to capture the ventricles16,17. The drive train comprised eight basic stimuli (S1-S1=330 ms) at twice the diastolic threshold, followed by a premature stimulus (S2). The S1-S2 interval was decreased from 250 ms initially by decrements of 10 ms and then 2 ms when approaching ERP. ERP dispersion was defined as the difference between the maximum and minimum values of the ERP at all six recording sites.
CONSTRUCTION OF ACTION POTENTIAL DURATION (APD) RESTITUTION CURVES
As described in our previous studies17,18, programmed stimulation was performed to construct APD restitution curves. A dynamic steady state pacing protocol (S1-S1) with a series of pulse trains at constant pacing cycle length was performed to obtain monophasic APD at LVM and RVM. The sites where APD was recorded were marked so that the protocols could be repeated at the same sites after interventions. The pulse train was delivered at an initial pacing cycle length just slightly shorter than the sinus cycle length and maintained for 30 seconds to achieve a steady state. After delivering each pulse train, the pacing cycle length was decreased in 10 ms steps until APD alternans occurred.
The monophasic action potential recordings were analysed using the LEAD 2000B workstation system (Jingjiang Inc., Chengdu City, China). All APDs were analysed at repolarisation levels from 10%, 50%, and 90% (APD10, APD50, and APD90, respectively). For construction of the APD restitution curve, APD90 and diastolic interval, which measured from the end of repolarisation time of the preceding beat to the activation time of the following beat, were measured at baseline and at each pacing cycle length. The APD restitution curves were constructed by plotting each APD90 against the preceding diastolic interval using Origin 7.5 software package (OriginLab Corporation, Northampton, MA, USA). The maximal slope (Smax) of the restitution curve was determined at the shortest DI. The Smax was calculated using a monoexponential equation as applied in our previous studies17,18.
EXPERIMENTAL DESIGN
The experimental design is depicted in Figure 1D. Twenty-four dogs were randomly assigned into control group (n=8), CSD group (n=8), and RSD group (n=8). In the eight control animals, only bilateral thoracotomies were performed to measure the electrophysiological parameters without further operations. Ventricular ERP and APD were determined at the following four time points: baseline, 0 min, 30 min, and 60 min after sympathetic interventions, while ventricular APD restitution curves were constructed at baseline and 60 min after the interventions in each group.
STATISTICAL ANALYSIS
All continuous variables were presented as mean±standard deviation. One-way ANOVA followed by a Tukey’s test was used to detect the differences in HR, systolic and diastolic BP, QT interval, QTc, ventricular ERP, APD, ERP dispersion and Smax among three groups at the same time point. The APD alternans cycle length was compared among three groups at the same time point by the Kruskal-Wallis test and followed by a Dunn’s test. SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was defined as p<0.05.
Results
EFFECTS ON HR, BP, QT INTERVAL AND QTC
The preprocedural values of HR, BP, QT interval and QTc were not significantly different among the three groups (Table 1). Sixty minutes after interventions, both CSD and RSD significantly reduced HR and prolonged QT interval without affecting BP and QTc when compared to the control group (Table 1).
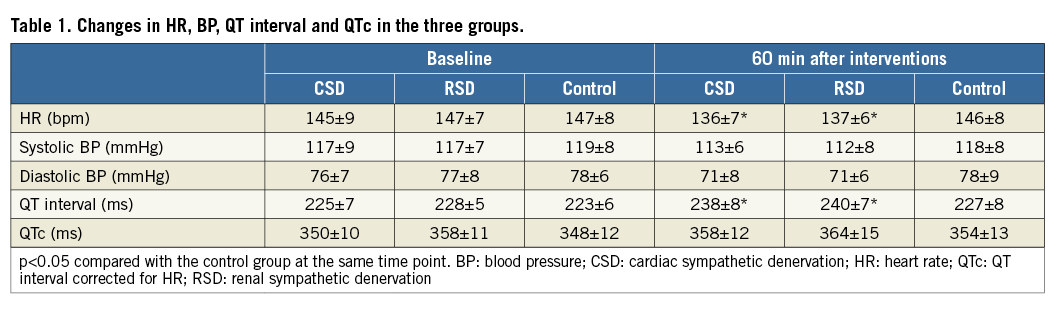
EFFECTS ON VENTRICULAR ERP AND APD
As shown in Figure 2 and Figure 3, at the 60 min time point, both CSD and RSD significantly prolonged the ventricular ERP, APD50, and APD90 at each recording site when compared to the control group. However, APD10 did not change significantly by either CSD or RSD. For example, baseline values of APD10 at the LVM site in the CSD group, RSD group and control group were 118±5 ms, 118±6 ms, 118±6 ms, respectively (p>0.05), while these values at the 60 min time point were 119±6 ms, 120±5 ms, and 119±7 ms, respectively (p>0.05). Meanwhile, the ventricular ERP dispersion was significantly decreased by both CSD and RSD when compared to the control group at the 60 min time point (Figure 4). However, no significant changes were seen between the CSD and RSD groups at the same time point (Figure 2-Figure 4).
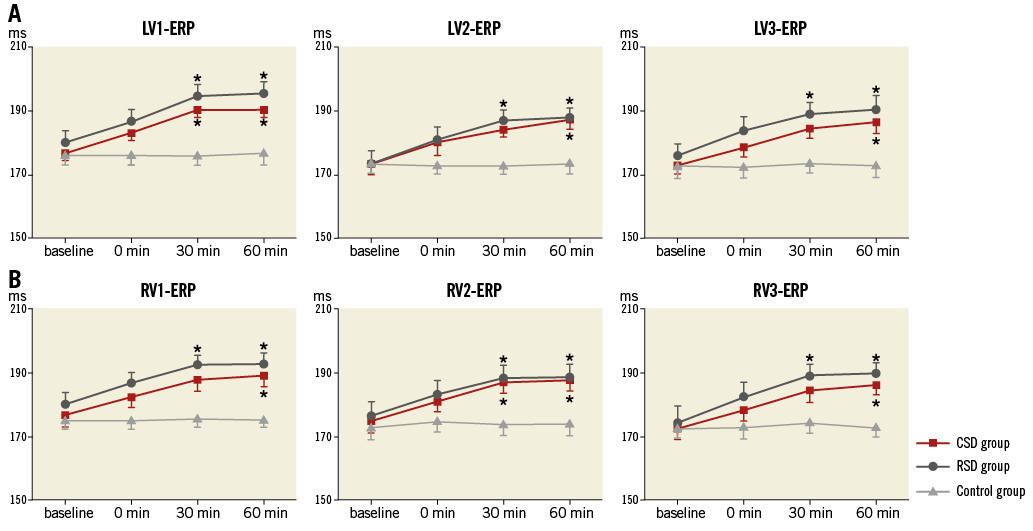
Figure 2. ERP in the three groups at four different time points. A) Left ventricular ERP. B) Right ventricular ERP. *p<0.05 compared with the control group at the same time point. CSD: cardiac sympathetic denervation; ERP: effective refractory period; LV: left ventricle; RSD: renal sympathetic denervation
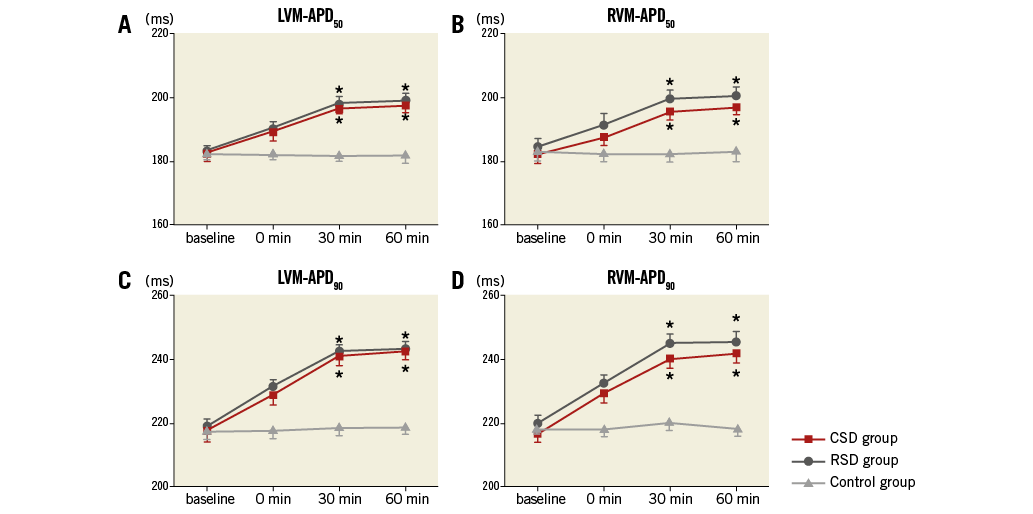
Figure 3. APD in the three groups at four different time points. A) & C) Left ventricular APD. B) & D) Right ventricular APD. *p<0.05 compared with the control group at the same time point. APD: action potential duration; CSD: cardiac sympathetic denervation; LVM: the median area of LV; RSD: renal sympathetic denervation; RVM: the median area of RV
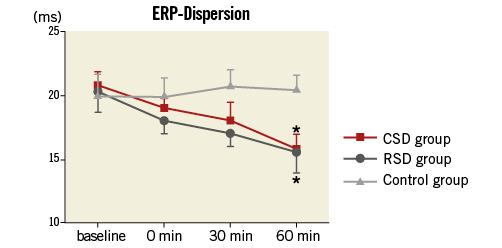
Figure 4. Ventricular ERP dispersion in the three groups at four different time points. *p<0.05 compared with the control group at the same time point. CSD: cardiac sympathetic denervation; ERP: effective refractory period; RSD: renal sympathetic denervation
EFFECTS ON VENTRICULAR APD RESTITUTION PROPERTIES
Figure 5A and Figure 5B are typical examples showing the effect of CSD or RSD on ventricular APD restitution curves at LVM and RVM in a dog. Both CSD and RSD caused an upward shift of the curve and a prolongation of the APD90 when compared with baseline at corresponding DI (Figure 5A, Figure 5B). For example, the APD90 in the LVM was increased from a baseline of 220 ms (218 ms) to 242 ms (239 ms) at the 60 min time point after RSD (CSD) at the same diastolic interval of 140 ms. The mean Smax was significantly lower at the 60 min time point in both the CSD and RSD groups than in the control group (Figure 5C, Figure 5D). However, Smax was not significantly different between the CSD and RSD groups at the same time point (Figure 5C, Figure 5D).
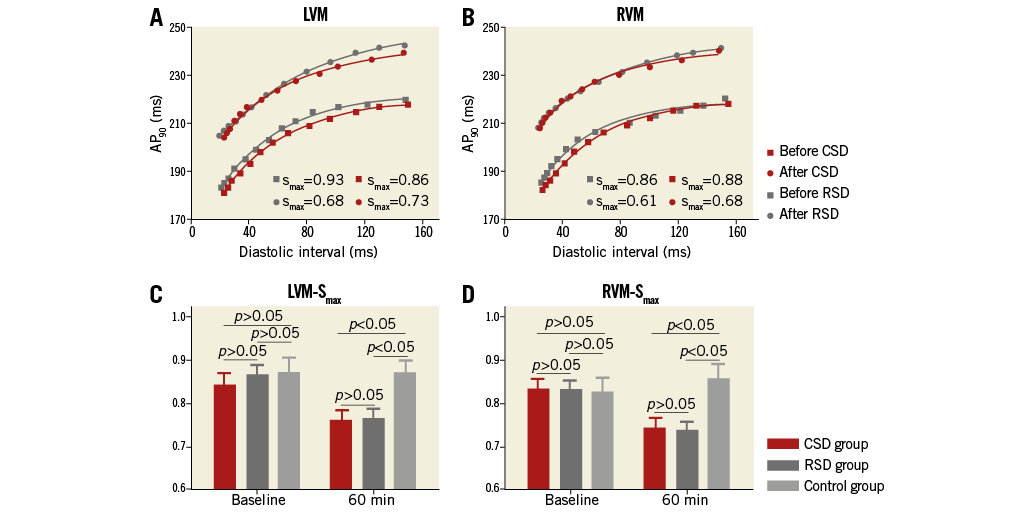
Figure 5. Typical examples of dynamic APD restitution curves before and 60 min after CSD or RSD. A) At LVM. B) At RVM. The APD restitution curves were upward shifted and flattened by ablation. A significant decrease was shown in Smax in both CSD and RSD groups when compared to control group at the time point of 60 min after intervention (C & D). APD: action potential duration; CSD: cardiac sympathetic denervation; LVM: the median area of LV; RSD: renal sympathetic denervation; RVM: the median area of RV; Smax: the maximal slope of APD restitution curve
EFFECTS ON VENTRICULAR APD ALTERNANS
Figure 6 shows the pacing cycle length at which APD alternans occurred before and after interventions. Compared with the control group, APD alternans occurred at significantly shorter cycle length in both the CSD and RSD groups at the 60 min time point, that is to say, both CSD and RSD suppressed APD alternans. Again, there was no significant difference between the CSD and RSD groups at the same time point (Figure 6).
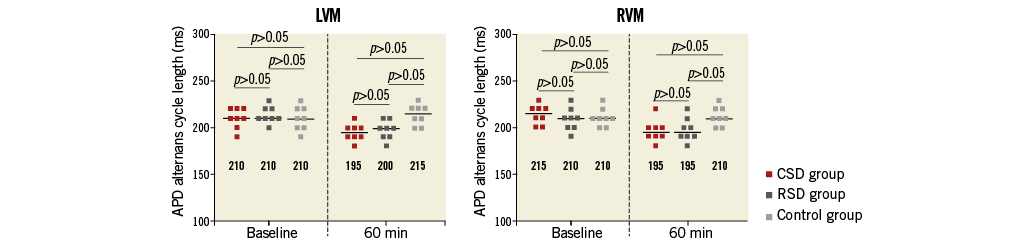
Figure 6. The pacing cycle length of APD alternans at LVM and RVM in the three groups. At the time point of 60 min, the pacing cycle lengths in both CSD and RSD groups were significantly shorter than in the control group. APD: action potential duration; CSD: cardiac sympathetic denervation; LVM: the median area of LV; RSD: renal sympathetic denervation; RVM: the median area of RV
Discussion
According to the restitution hypothesis19-21, a steep restitution slope promotes while a flattened restitution slope prevents the occurrence of oscillations and wave breaks which facilitate fatal VA, e.g., ventricular fibrillation. APD alternans which occurs more easily with a steep restitution slope is a predictor of ventricular fibrillation19-21. Moreover, a greater dispersion of refractoriness is associated with conduction block and re-entry, also promoting ventricular fibrillation22. The major finding of this study is that CSD and RSD similarly flatten the APD restitution curves, suppress the occurrence of APD alternans, and decrease the ventricular ERP dispersion, suggesting that sympathetic denervation of kidney and heart might similarly increase the ventricular electrophysiological stability.
We also found that both CSD and RSD prolonged QT interval, ventricular ERP and APD. The prolongations of QT interval and APD were considered as useful, but not the sole or optimal, markers predicting the development of a life-threatening arrhythmia, such as torsades de pointes23. In this study, we calculated the QTc and found that the QTc did not change significantly, suggesting that the QT and APD prolongation is probably caused by CSD- or RSD-induced reduction of HR, and may not be a true prolongation of ventricular repolarisation.
It is well established that cardiac sympathetic nerves, especially in the LSG, play an important role in the modulation of ventricular electrophysiology24 and arrhythmias25. Previous studies24,26 have shown that LSG stimulation significantly shortens ventricular ERP and APD, while LSG block or resection prolongs them, consistent with our study. In a canine model of sudden cardiac death25, direct nerve activity recording from the LSG showed that increased LSG nerve activities were immediate triggers of VA and sudden cardiac death. Chen and his colleagues found that infusion of nerve growth factor27 or applying chronic electrical stimulation28 to the LSG could cause cardiac sympathetic nerve sprouting and increase the incidence of VA. These studies suggested that hyperactivity of LSG contributes to the pathogenesis of VA and sudden cardiac death. Therefore, LSG denervation may have antiarrhythmic and antifibrillatory properties that potentially provide a therapeutic approach to the prevention of sudden cardiac death. Indeed, left CSD by ablation or resection of LSG provides the greatest degree of protection against drug-refractory malignant VA, such as electrical storm1, congenital long QT syndrome2, and catecholaminergic polymorphic ventricular tachycardia3. Although it is known that CSD has protective effects on VAs, its effects on ventricular electrophysiological properties have not been fully clarified. We report in the present study that CSD performed by LSG ablation may increase ventricular electrophysiological stability, providing a possible mechanism underlying its antifibrillatory actions.
Recently, the RSN, which has been identified as a major mediator of systemic sympathetic tone, has become an interesting target for intervention. Numerous studies have demonstrated that RSD can reduce whole body sympathetic activity29 and exert a therapeutic effect in diseases associated with hypersympathetic tone11-13.
Interestingly, cardiac electrophysiology and arrhythmias can also be affected by RSD. Basic30 and clinical31 studies show that RSD results in a reduction in heart rate and AV-conduction velocity. In a pig model of obstructive sleep apnoea32, RSD and beta-blockers have comparable effects in suppressing negative tracheal pressure-induced atrial electrical remodelling. In a small human study33, resistant hypertension patients who received pulmonary vein isolation (PVI) combined with RSD were associated with significant reductions in average systolic and diastolic blood pressure, whereas those who received PVI only did not show any significant improvement in blood pressure. Moreover, at one-year follow-up, 69% of patients who received PVI+RSD no longer had atrial fibrillation recurrences, compared to 29% of those who received PVI only33. Recent studies suggest that RSD, in addition to exerting a protective effect on atrial arrhythmias, also has therapeutic value in the treatment of VAs. Recent studies by Linz et al34 and from our group35 have demonstrated that RSD was able to reduce ventricular premature contraction and ventricular fibrillation induced by acute myocardial ischaemia. Ukena et al7 first provided clinical evidence that RSD could reduce the episodes of ventricular tachyarrhythmias in patients with dilated cardiomyopathy suffering from electrical storm. Recently, several studies8-10 have focused on the therapeutic role of RSD in ventricular tachycardia storm. However, the actions of RSD on ventricular electrophysiological properties remain unclear. In the present study, we found that RSD significantly prolonged ventricular ERP and APD, increased ventricular electrophysiological stability, indicating that RSD might exert a protective effect on VAs, consistent with the basic34 and clinical7-10 findings described above.
Another main finding of our study is that the effects on ventricular electrophysiological properties by CSD and RSD are similar. The mechanisms of this finding are unknown but appear to arise from modulation of cardiac sympathetic tone by both CSD and RSD. CSD, which directly destroys the cardiac sympathetic neurons in LSG, has been shown largely to prevent norepinephrine release in the heart36,37. Clinical studies14,29 in patients with resistant hypertension have proven that RSD is able to reduce renal norepinephrine spillover by 47% as well as the whole body sympathetic activation by 37%. A recent study from our group showed that 3 hrs of electrical stimulation of left RSN significantly increased neuronal activity and neural remodelling in the LSG and facilitated ischaemia-induced VAs, suggesting a potential link between RSN and LSG38. According to this study, we speculate that RSN can modulate central sympathetic activity, which in turn affects efferent neural inputs to the LSG. Therefore, it is possible that RSD which has been shown to reduce central sympathetic activity29 may decrease sympathetic inputs to the LSG. Taken together, we presumed that both RSD and CSD could reduce cardiac sympathetic tone, which might be the possible mechanisms underlying their similar effects on ventricular electrophysiological properties.
Study limitations
Firstly, the whole study was performed under general anaesthesia. Anaesthesia has been shown to alter cardiac electrophysiological properties. Secondly, in this study we only included healthy animals and we did not evaluate the effect of the interventions on ventricular electrophysiology in a pathological model, e.g., chronic heart failure. However, a recent study by Guo et al39 showed that ventricular ERP, mean corrected QT interval, ERP dispersion and QT dispersion were significantly increased in a canine model of pacing-induced heart failure and were attenuated by RSD, suggesting a beneficial effect of RSD on ventricular electrophysiological remodelling in this canine model. Thirdly, this is only an observational study and lacks mechanistic investigation. Although we suggest that reductions of cardiac sympathetic tone by both CSD and RSD may be related to their similar effects on ventricular electrophysiological properties, nerve activity recording is needed to provide direct evidence. Fourthly, we only investigated the short-term effects of CSD/RSD on ventricular electrophysiology. Further studies will be needed to elucidate the long-term effects and to show whether re-innervation will happen after ablations. Lastly, we performed RSD via the retroperitoneal approach in this study; however, it was performed by the inter-arterial approach in clinical practice. Whether the results might be applicable to an intra-arterial approach remains to be elucidated by further studies.
Conclusions
We demonstrated that sympathetic denervation of heart and kidney induced similar electrophysiological effects in ventricles.
| Impact on daily practice Cardiac sympathetic denervation (CSD) has been shown to decrease cardiac events significantly in patients at high risk for sudden cardiac death (SCD), e.g. long QT syndrome. However, the clinical use of CSD is limited to only a few centres, largely due to the complexity of the surgery and the potential complications associated with it. The present study showed that CSD and renal sympathetic denervation (RSD) induced similar electrophysiological effects in ventricles. In future, RSD may serve as a less invasive alternative to CSD for the prevention of SCD. |
Funding
This work was supported by grants from the National Natural Science Foundation of China (81270339; 81300182; 81370281; 81270250; 81300181), Science and Technology Research Project of Wuhan (201306060201010271), Natural Science Foundation of Hubei Province (2013CFB302), and Fundamental Research Funds for the Central Universities (2012302020206; 2042012kf1099; 2042014kf0110).
Conflict of interest statement
The authors have no conflicts of interest to declare.

