Abstract
Chronic heart failure is associated with sympathetic activation characterised by elevated circulating norepinephrine levels linked to cardiovascular morbidity and mortality. Norepinephrine induces phenotype changes of the cardiomyocyte, fibrosis and β-adrenergic signal transduction defects implicated in the dysregulation of contractility. Renal denervation reduces left ventricular hypertrophy and improves diastolic dysfunction, partly blood pressure independently. Also, exercise tolerance and cardiac arrhythmias are positively influenced. Furthermore, there is evidence that common comorbidities like sleep apnoea, metabolic disease and microalbuminuria are improved following renal denervation. The available evidence suggests performing randomised controlled trials to scrutinise whether renal sympathetic denervation might be able to improve morbidity and mortality in chronic heart failure with preserved or reduced ejection fraction.
Introduction
During regulation of sympathetic drive the kidney increases sympathetic outflow by afferent nerves1,2, targeting organs like the heart, vessels, liver and in turn also the kidneys3. Excessive activation of the sympathetic nervous system is associated with the development of hypertension4, sleep apnoea5, metabolic disease6 and liver cirrhosis7. In the myocardium, the effects of sympathetic activation are mainly stimulated by beta1- and beta2-adrenoceptors8. After excessive stimulation, as occurs in severe hypertension9 and heart failure10, the beta-adrenergic signalling system is impaired potentially contributing to the pathophysiology of heart failure and hypertensive cardiomyopathy11. Furthermore, elevated sympathetic tone might be relevant to increased adverse clinical outcome in heart failure, because elevated circulating concentrations of norepinephrine are associated with poor outcomes12,13. Here we describe the rationale and potential applications of reducing sympathetic drive by catheter-based renal denervation in chronic heart failure.
Adrenergic signalling and changes in heart failure and cardiac hypertrophy
Chronic heart failure is characterised by an activation of the sympathetic nervous system as evidenced in several experimental models10 and in patients with heart failure12,13. Excessive stimulation of beta-adrenergic receptors by norepinephrine leads to receptor phosphorylation and consequent downregulation (Figure 1)14. In the failing heart, beta-adrenoceptor downregulation is well established8,15. It has been closely associated with the reduction of the effect of beta-adrenergic agonists16. Since beta-adrenergic-independent positive inotropic agents also have a reduced effect in failing myocardium17, there is evidence that post-receptor trends are relevant: these have been identified as an upregulation of inhibitory G-proteins (Giα)17,18, an upregulation of beta-arrestins and beta-adrenergic receptor kinase19,20. Similar results have been observed in hypertensive cardiac hypertrophy11,21,22 as well as in hypertensive cardiomyopathy23. These effects which are sensitive to antihypertensive therapy24-26 have been reported to occur in prehypertensive cardiac hypertrophy27, and are also sensitive to treatment with inhibitors of the renin-angiotensin system28 at even subhypertensive doses29. Besides an increase of circulating catecholamine concentrations12,13, norepinephrine is released from the failing heart30, which might at least in part be attributed to a reduction of norepinephrine uptake in vivo31 and in vitro32. Therefore, sympathetic activation directly influences the cardiac phenotype and limits the regulation of contractility in severe hypertensive cardiac hypertrophy11,21-29, but also in chronic heart failure16-18,32.
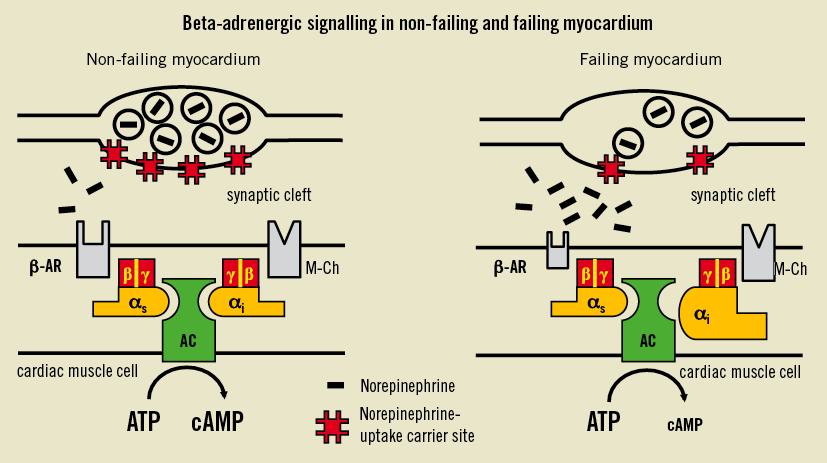
Figure 1. Scheme of functional defects in the failing human myocardium. Downregulation of cardiac beta-adrenoceptors (β-AR) and an increase of inhibitory guanine binding proteins (Giα) lead to a reduced formation of the intracellular second messenger cyclic adenosine monophosphate (cAMP). In addition to these postsynaptic changes, there could be a presynaptic defect in the sympathetic nerve system consisting of a reduced density of norepinephrine uptake carrier sites. The consequence could be an increase in the concentration of norepinephrine in the synaptic cleft37. AC: adenylyl cyclise; ATP: adenosine triphosphate; M-CH: m-cholinoceptors; αi (αs): Gi (Gs)-protein subunit alpha; β (γ): G-protein subunit beta (gamma). Modified from 32.
Change of the cardiac phenotype after renal denervation
Hypertensive cardiomyopathy is characterised by a high prevalence of fibrosis, diastolic dysfunction, and myocyte hypertrophy, which are predictive of the development of chronic heart failure33. There is evidence that the direct effects of the sympathetic nervous system influence these processes34. The degree of these changes is associated with comorbidities and mortality35,36 and is sensitive to antihypertensive treatments with significant variability in drug classes36,37.
Following renal denervation, it was observed that left ventricular mass index at one and six months was significantly reduced38. Interestingly, this was accompanied by a reduction of left ventricular filling patterns as judged from an improvement of lateral E/E’ in echocardiography (Figure 2)38. In this population, 25% fulfilled the echocardiographic criteria of heart failure with preserved ejection fraction according to the Guidelines of the European Society of Cardiology39. Since the antihypertrophic effect occurs quite early at one month38, it was suggested that a blood-pressure-independent effect might play a certain role40. If the changes observed herein are indeed significant predictors and surrogates of outcomes, these findings are a plea for a clinical study addressing heart failure, in particular in individuals with heart failure with preserved ejection fraction.
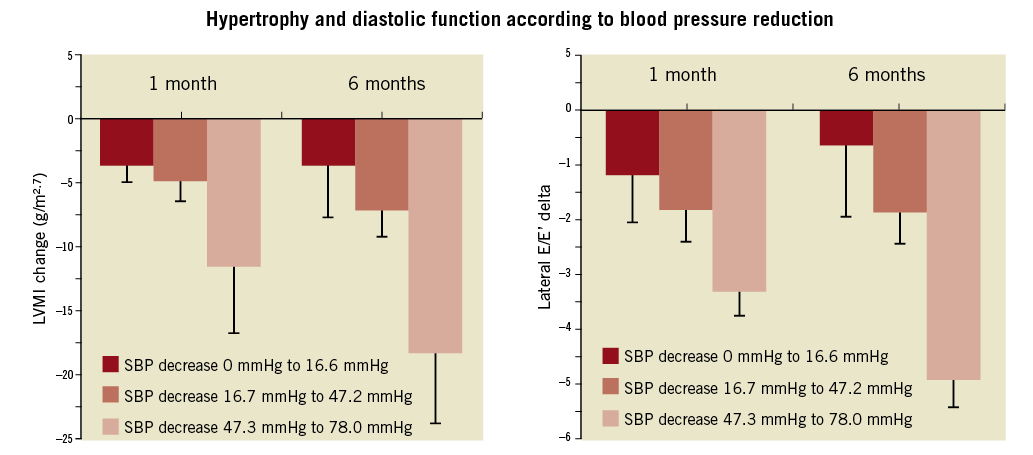
Figure 2. Changes of left ventricular mass index (LVMI) and improvement of diastolic function according to lateral E/E’ (right) after one and six months, respectively, according to tertiles of blood pressure reduction. Note that ventricular mass changes and improvement of diastolic dysfunction occur in non-responders and those with low blood pressure response quite early at one month38.
The evidence on effectiveness in patients with systolic heart failure is sparse. Several studies and investigations are planned to address whether the effects on symptoms and outcome surrogates can be improved by renal denervation. One early pilot study in seven patients showed a trend towards improvement of exercise tolerance41, which was the preparation for an outcome trial (REACH, NCT 01639378). Several other studies such as Symplicity-CHF and RE-ADAPT-CHF are addressing similar endpoints investigating cardiac function and structure as well as exercise tolerance.
The effect on comorbidities
Heart failure is associated with significant non-cardiac comorbidities affecting outcome39. Renal impairment is present in about 50% of patients with significant heart failure39 and is associated with further complications42. Influencing the cardio-renal syndrome of heart failure has the potential to improve outcomes beneficially. However, the interventions could also place these patients at risk due to the application of contrast dye and potential changes of intrarenal haemodynamics. In a preliminary study, microalbuminuria was observed to be reduced by renal denervation43, potentially involving an improvement of intrarenal resistive indices43. Furthermore, it was shown that the procedure is safe in patients with moderate to severe kidney disease44. Associated with renal disease, metabolic syndrome is likewise a problem. Again, about 50% of patients with symptomatic heart failure suffer from insulin resistance and diabetes mellitus type 239. Renal denervation has been shown to reduce fasting glucose, insulin and C-peptide concentration and to improve glucose tolerance test45. This finding is pathophysiologically plausible, because diabetes type 2 and insulin resistance are closely associated with sympathetic activation46,47 and represent an important comorbidity in heart failure39, also in severe hypertension3. A significant number of heart failure patients suffer from sleep apnoea49. Renal sympathetic denervation has been shown to improve sleep apnoea severity (measured by apnoea/hypopnoea index) and glycaemic control50 providing evidence that these two conditions are also linked to each other. Finally, arrhythmias are a common problem in heart failure (leading to sudden death) with the necessity for device implantation39. Experimentally, renal denervation was able to reduce susceptibility to atrial fibrillation51,52. In a “first-in-man” experience, it was shown that, in patients with cardiomyopathy and electrical storm, renal denervation was associated with an improvement and interruption of this life-threatening complication53.
Summary
Heart failure is characterised by sympathetic overactivity, which is related to cardiovascular mortality and morbidity as well as directly changing the phenotype of the failing heart concerning cellular beta-adrenergic signal reduction and myocardial hypertrophy and fibrosis. Since all these factors are associated with outcomes, these findings urgently suggest performing a randomised controlled trial with renal denervation to provide evidence for outcome improvement in heart failure with preserved and reduced ejection fraction. The potential of this treatment to improve clinical care of this malignant condition is emphasised by the beneficial effects on the cardiac comorbidities of heart failure such as sleep apnoea, arrhythmia and metabolic syndrome which can be taken as surrogates for cardiovascular outcomes.
Acknowledgements
Scientific work is supported by the Deutsche Forschungsgemeinschaft (M. Böhm, C. Ukena), the Deutsche Hochdruckliga (F. Mahfoud, S. Ewen) and the Deutsche Gesellschaft für Kardiologie (M. Böhm, D. Linz, F. Mahfoud).
Conflict of interest statement
All authors received research support from Medtronic, Ardian and St. Jude.

