Abstract
The prevalence of chronic kidney disease (CKD) and end-stage renal disease continues to increase worldwide. Hypertension and diabetes are recognised as two major factors contributing to further progression of CKD. Importantly, progressive renal impairment increases cardiovascular morbidity and mortality. Despite advances in pharmacological antihypertensive and anti-diabetic approaches, the alarming number of patients developing nephropathy indicates the failure of the available treatment strategies. The relevance of sympathetic activation for the development and progression of chronic kidney disease is well established. Likewise, progressive renal failure results in exaggerated sympathetic activation leading to a vicious cycle, providing the rationale for the use of renal denervation to modulate directly the mechanisms underlying disease progression. While initial data on the safety and effectiveness of the procedure to lower BP were obtained in patients with resistant hypertension and preserved renal function, there are now preliminary data to suggest that this approach can also be applied safely in patients with stage 3-4 CKD. Similarly, first reports applying renal denervation in patients with end-stage renal disease on dialysis demonstrate favourable effects. If appropriately designed clinical trials can confirm these initial observations, renal denervation may become a valuable new treatment option for the large cohort of patients with CKD.
Introduction
Chronic kidney disease (CKD) contributes considerably to the global burden of cardiovascular (CV) morbidity and mortality1. Patients with uncontrolled blood pressure (BP) and reduced glomerular filtration rate (GFR) are at high risk of developing CV disease and sudden cardiac death. The risk of CV morbidity and mortality is even higher than that of developing end-stage renal disease (ESRD)2-5.
Despite the availability of safe and effective pharmacological strategies to lower BP and glucose levels, the prevalence of CKD6 and ESRD7 is growing, primarily attributable to uncontrolled BP8 and diabetes9. Recent estimations by the National Health Institute and United States Renal Data System indicate that one in ten American adults suffers from CKD, an increasing problem particularly in those aged over 607. Patients with resistant hypertension (RH), defined as the failure to attain target BP with triple antihypertensive therapy including a diuretic10, are particularly vulnerable to CKD as demonstrated by a twofold increase in the risk for adverse CV events11. In this context, novel strategies are required to tackle the ever growing burden of CKD.
Here, we briefly summarise the evidence for a causal role of sympathetic activation in renal disease development, and we review the recent clinical developments using therapeutic catheter-based renal sympathetic nerve ablation in hypertensive patients with CKD and ESRD.
Role of the sympathetic nervous system in chronic kidney disease
The contribution of the sympathetic nervous system to the development, progression and complication of CKD has been extensively investigated in experimental and human studies12-16. The first studies demonstrated increased levels of plasma norepinephrine in patients with CKD17,18; however, altered re-uptake and metabolism of norepinephrine may also affect neurotransmitter levels in the presence of impaired renal function19. The important contribution of neural mechanisms to the BP elevation typically present in renal failure patients was initially demonstrated by the substantial decrease in BP following blockade with atropine, prazosine and propranolol20. Accordingly, therapeutic adrenergic blockade with clonidine21 or debrosoquine22 resulted in a reduction of BP and catecholamines in renal failure patients. Further evidence for increased sympathetic activation came with the introduction of microneurography to measure sympathetic activity in humans directly. Augmented sympathetic activation to the peripheral vasculature as evidenced by increased muscle sympathetic nerve activity (MSNA) has been found in various stages of CKD23,24, including mild to moderate CKD25 and polycystic kidney disease even in the presence of normal glomerular filtration rate (GFR)26. Furthermore, sympathetic excitation has also been shown to be elevated in early phases of chronic renal failure and the magnitude of sympathetic drive increases with disease progression27. Sympathetic activation contributes substantially to the poor prognosis in CKD and is a predictor of CV and total mortality in patients with ESRD28,29.
Several other closely interrelated mechanisms including activation of the renin-angiotensin system30,31, hyperleptinaemia32, altered regulation of the nitric oxide system33, tonic chemoreflex activation34 and others are likely to contribute to sympathetic activation in patients with renal disease (Figure 1).
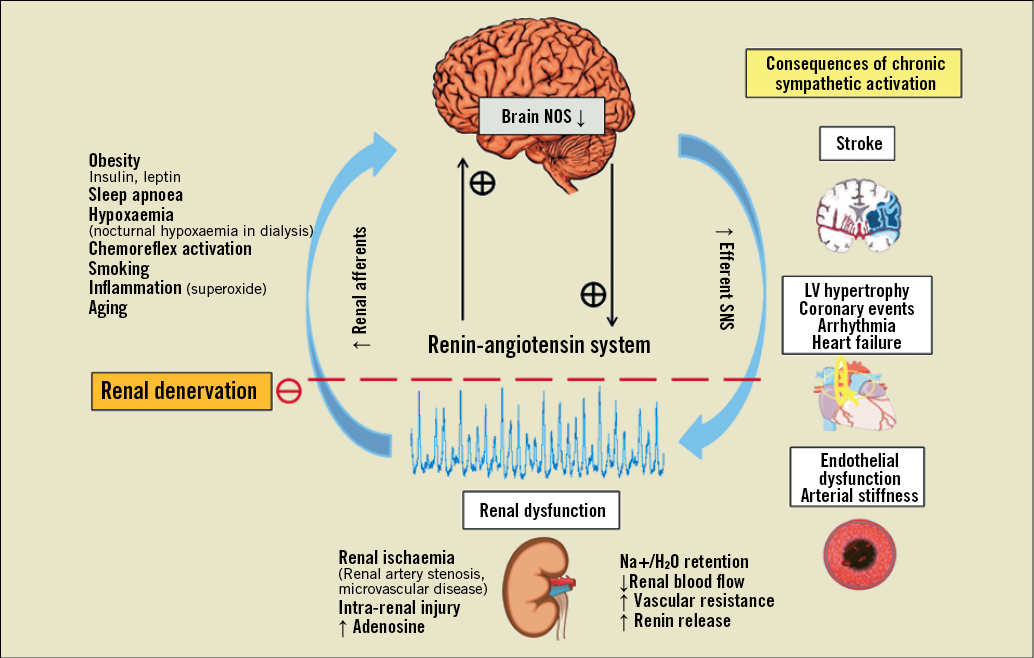
Figure 1. Mechanisms of sympathetic activation in renal disease.
The human kidneys are richly innervated by sympathetic efferent and sensory afferent fibres, with the latter enhancing sympathetic drive via a centrally integrated response to activation of renal chemo- and mechano-receptors. While in healthy subjects activation of afferent renal sensory fibres exerts an inhibitory influence on efferent renal nerve activity via reno-renal reflexes, the presence of intra-renal ischaemia and/or renal injury activates afferent renal nerves that can potentiate central sympathetic outflow to the periphery with potential adverse consequences if sustained over longer periods of time19,35, as illustrated in Figure 1. Stimulation of efferent renal sympathetic nerves increases sodium reabsorption, reduces renal blood flow, and increases renin release with subsequent stimulation of the renin-angiotensin-aldosterone system36 (Figure 1).
The contribution of afferent sensory renal fibres originating from the kidney and projecting to the brainstem to activate hypothalamic centres and thereby increasing central sympathetic outflow has been convincingly demonstrated in experimental studies37-40. The BP elevation and deterioration of renal function present in rats after 5/6 nephrectomy37 could be prevented by bilateral dorsal rhizotomy38. Accordingly, the increase in norepinephrine (NE) turnover in the posterior and lateral hypothalamic nuclei was attenuated following bilateral dorsal rhizotomy38. RDN has been shown to prevent sympathetic excitation and retard the development of hypertension in experimental models39. Evidence for a neurogenic contribution to renal hypertension has also been derived from a rat model of renal artery stenosis40.
In humans, RDN was first performed in the early 1920s to treat nephralgia and pain-related hydronephrosis41. Several case reports from the mid 1930s evaluated this procedure for reflex anuria, early tuberculosis of the kidney, nephritis, painful nephroptosis or painful motility of the renal pelvis. Evidence from studies in humans supports the concept of afferent signals emanating from the native failing kidney to stimulate central pathways and enhance sympathetic outflow15,21. Accordingly, bilateral nephrectomy has been shown to reduce or even normalise MSNA in patients on haemodialysis23. Furthermore, renal transplant recipients, in whom MSNA remained high despite a well-functioning transplant with normal creatinine levels, experienced a reduction in MSNA only if bilateral nephrectomy was performed, suggesting that the native kidneys, which are in situ in the majority of haemodialysis patients, are the cause of an activated sympathetic nervous system via afferent signalling24. In haemodialysis patients with refractory hypertension bilateral nephrectomy has also been shown to improve BP control and even been suggested as the best treatment for this patient cohort42.
Given that the contribution of renal nerves is well established in the initiation, development and maintenance of elevated BP in renal failure patients43, the interruption of efferent and afferent renal pathways may potentially restore autonomic imbalance, and reduce both renal sympathetic outflow and arterial BP in this scenario.
Renal denervation in patients with preserved kidney function
The clinical experience currently available with catheter-based renal sympathetic nerve ablation is primarily derived from studies in patients with treatment-resistant hypertension and office SBP≥160 mmHg (≥150 mmHg in the presence of type 2 diabetes) who failed to attain target BP with triple antihypertensive therapy. The available evidence indicates that catheter-based renal denervation (RDN) has a favourable safety profile and results in a sustained BP lowering effect in patients with RH and preserved renal function (eGFR ≥60 ml/min/1.73 m2) as demonstrated by the results from the Symplicity HTN-1 and HTN-2 trials44-47.
In the Symplicity HTN-2 trial no deterioration in renal function as assessed by serum creatinine, cystatin C and estimated GFR was observed at six months post procedure45. Following the longer- term observation of the proof-of-concept Symplicity HTN-1 trial46, creatinine-based estimated GFR remained unaltered in patients who completed 12-month follow-up, but was reduced by -16.0 mL/min per 1.73 m2 at 24 months post procedure in 10 patients for whom the data were available. Importantly, in patients without newly added spironolactone or other diuretics (n=5) after 12 months post RDN, eGFR changed by -7.8 mL/min per 1.73 m2 for an annualised change of -3.9 mL/min per 1.73 m2. No patient developed stage 4 CKD or required dialysis therapy. A recent study evaluating the influence of RDN on renal haemodynamics, renal function and urinary albumin excretion ratio in a total of 88 patients with RH revealed that, in addition to BP and pulse pressure decrease, a parallel reduction in renal resistive index from 0.691±0.01 at baseline to 0.674±0.01 and 0.670±0.01 (p<0.05) at three and six-month follow-up, respectively, was evident following the procedure48. The proportion of patients with normal urinary albumin excretion increased by 5% and 12%, whereas the proportion of patients with microalbuminuria and macroalbuminuria decreased by 10% and 23%, at three and six-month follow-up, respectively48. Of note, in line with previous findings, eGFR remained unchanged six months post procedure, indicative of a favourable safety profile and potentially beneficial effects on renal function in patients with RH.
Renal denervation in chronic kidney disease
Recently, a small proof-of-concept study investigated the safety and effectiveness of RDN in patients with CKD and associated drug-resistant hypertension49-53. This first-in-human pilot study included a total of 15 patients with stage 3-4 (eGFR: 15 and ≤45 ml/min/1.73m2) CKD and demonstrated the safety and feasibility of RDN with effective BP response in high-risk renal patients49. Office systolic and diastolic BP levels were significantly reduced by -34±13/-14±13,-25±20/-11±10, -32±18/-15±12, -33±20/-19±20 mmHg at one, three, six, and 12 months following RDN, respectively (p<0.001). Of potential clinical significance was a reduction in maximum SBP following RDN, given the known association between maximum SBP and risk of stroke54. Additionally, RDN decreased night-time ambulatory BP (p=0.01) three months post procedure, resulting in a significant restoration of a more physiological dipping pattern which may further improve patients’ outcomes. However, average daytime and mean 24-hour BP were not significantly reduced following the procedure.
An additional important observation from this pilot study was the absence of deterioration of renal function assessed by estimation of GFR based upon serum creatinine or cystatin C levels, and plasma creatinine, cystatin C or urea levels with short-term (seven and 28 days) and long-term (up to 12 months) follow-up. Changes in creatinine-based eGFR in individual patients following the procedure are shown in Figure 2. No intra- or periprocedural problems with either iodine or CO2-angiography to minimise contrast exposure were encountered following the procedure. No evidence of aggravation of renal impairment was observed three months post procedure as demonstrated by measuring an effective renal plasma flow and kidney retention index in both kidneys using 99mTc-MAG3 scanning. Moreover, the observed improvement in augmentation index (AIx) associated with RDN may be clinically relevant in patients with CKD given that not only the presence but also the extent of arterial calcifications are strong predictors of CV and total mortality in ESRD55.
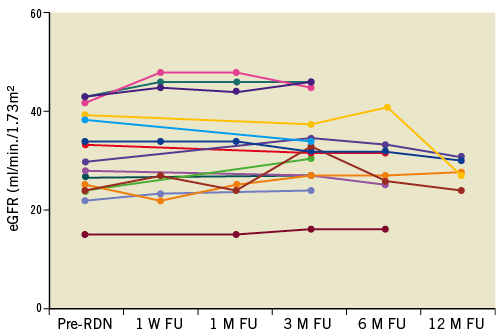
Figure 2. Individual changes in creatinine-based estimated glomerular filtration rate (eGFR) before renal denervation (pre-RDN), at 1 week (W), and 1, 3, 6, 12-month (M) follow-up (FU). Reproduced with permission from 49.
Further interesting yet unsubstantiated observations derived from this study were a tendency towards gradual increases in serum haemoglobin levels in treated patients49. Given the increased prevalence of anaemia with worsening of renal function56 and the direct link to CV57 and cerebrovascular events58, this finding may bear clinical relevance and merits further investigation. Furthermore, there was a trend towards a gradual decrease in plasma brain natriuretic peptide (BNP) levels, urinary albumin-to-creatinine ratio, proteinuria, and glycated haemoglobin in CKD patients after RDN, as reported in previous studies59-61. These findings indicate that ablation of renal nerves could be a promising approach to attenuate disease progression.
Renal denervation in end-stage renal disease
The feasibility of RDN in ESRD was recently tested in an initial proof-of-concept pilot study including a total of 12 patients with ESRD and uncontrolled BP50. Patients had various underlying causes of ESRD including nephrosclerosis (n=4), glomerulopathies (n=5), IgA nephropathy (n=1), nephrolithiasis (n=1) and bilateral atrophic kidneys of unknown origin (n=1) and were on dialysis for an average duration of 3.6±2.6 years prior to RDN. RDN could only be performed in nine patients, whereas the remaining three patients had atrophic renal arteries, the diameter of which was less than 4 mm, preventing the appropriate positioning of the treatment catheter for adequate energy delivery. In the nine treated patients, average office systolic BP decreased from 166±16.0 to 148±11, 150±14, and 138±17 mmHg at three, six, and 12-month follow-up, respectively (Figure 3A and Figure 3B), whereas in untreated patients (n=3) BP remained unchanged (176±7 vs. 172±6 mmHg) at three-month follow-up. Twenty-four-hour ambulatory BP measurements obtained in a limited number of patients (n=5) revealed a significant reduction in systolic BP at three months without changes in diastolic BP. One patient had an increase in systolic and diastolic BP after the procedure (Figure 3B).
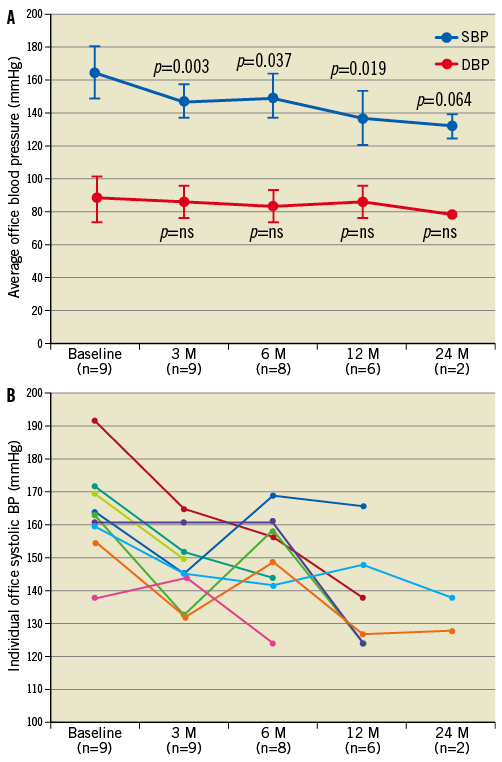
Figure 3. Average office systolic (SBP) and diastolic (DBP) blood pressure (A) and individual changes in office SBP (B), at baseline and at 3, 6, 12 and 24-month (M) follow-up (FU). Reproduced with permission from 50.
There was also a decrease in sympathetic neural outflow as demonstrated by reduced renal noradrenaline (NA) spillover directly after bilateral RDN and a substantial reduction in MSNA at follow-up in the two patients in whom assessment of sympathetic activation was obtained. The average number of antihypertensive drugs following RDN was reduced from 4.2±1.9 (n=9) at baseline to 4.0±1.9 (n=9), 3.7±2.3 (n=7), and 2.2±1.0 (n=5) at three, six, and 12-month follow-up, respectively, in this patient cohort.
Of particular interest amongst the ESRD patients treated by RDN was one patient who received a kidney transplant about four months after his bilateral RDN procedure50. One month prior to his renal transplant and three months after the RDN procedure BP was already substantially reduced (from 156/95 to 133/81 mmHg) accompanied by a reduction in renal NA spillover by ~22% and total body NA spillover by 15%. MSNA was significantly reduced and returned to normal at 12-month follow-up and was maintained after 33 months post procedure. Interestingly, despite substantial weight gain following transplantation (from 81.3 to 89.1 kg) and the use of immunosuppressive drugs to prevent rejection of the kidney transplant, BP control was maintained at 33-month follow-up (134/78 mmHg). BNP level was also reduced three months after RDN.
A substantial reduction in MSNA and renal NA spillover following RDN was also observed in the second patient50. In addition to the office BP fall post procedure, the reversed dipping pattern evident at baseline 24-hour BP profile was changed into a normal dipping pattern at three months which was sustained at 24 months. Consistent with data obtained in the previous patients, BNP was also reduced over time.
Similarly, in a recent case report, a 39-year-old ESRD patient with nephrosclerosis due to malignant hypertension despite taking five antihypertensive drugs52 experienced improved office BP control (from 180±15/105±11 to 155±14/90±10 mmHg) and reduced renin (from 13.12 to 11.06 ng/mL/h) and angiotensin-converting enzyme activity (from 22.62 to 14.94 IU/L) at one-month follow-up.
While very preliminary in nature, this first clinical experience with RDN in ESRD patients points towards its potential usefulness to lower BP and perhaps reduce CV risk in these high-risk patients, but clearly requires confirmation from appropriately designed clinical trials.
Renal denervation in polycystic kidney disease
One of the most common genetic disorders commonly progressing to ESRD is polycystic kidney disease (PKD). With the gradual enlargement of renal cysts over time, the presence of elevated BP is the most frequent clinical manifestation. Fluid-filled renal cysts may stretch renal tubules leading to ischaemia with subsequent activation of the afferent renal sensory fibres and consecutive development of elevated BP62. Chronic and often difficult to manage flank, abdominal or back pain also mediated via afferent renal nerve fibres is not uncommon in autosomal dominant polycystic kidney disease (ADPKD) patients. Amongst several interventions to treat chronic pain that is often unresponsive to long-term narcotic regimens, laparoscopic RDN has been shown to relieve cyst-induced pain substantially63. In this context, a recently published case report of a 58-year-old female with treatment-resistant hypertension and PKD-related chronic pain found clinical improvement of pain symptoms after RDN53. The patient had a five-year history of chronic flank pain presumably related to the presence of bilateral chronic renal cyst associated with ADPKD. While RDN was performed to treat the patient’s refractory hypertension, RDN was also associated with a rapid resolution of her pain shortly after the procedure, and the absence of pain was maintained at 12-month follow-up. Office systolic and diastolic BP (from 170/108 to 126/74 mmHg) as well as corresponding mean 24-hour ambulatory BP (from 165/99 to 116/75 mmHg) were substantially improved three months following RDN. In view of the often limited success with long-term analgesic regimens for chronic pain therapy and the associated risk of analgesia-induced nephropathy, RDN may represent a novel valuable tool for pain relief in PKD-induced pain syndromes. Clearly, this is a single patient report and needs to be interpreted with caution. Whether RDN may indeed be useful to treat chronic pain related to ADPKD and/or other conditions pertaining to severe pain including loin pain haematuria syndrome requires further investigation.
Renal denervation in renovascular hypertension
While renovascular hypertension is a common secondary cause of hypertension, renal revascularisation as a therapeutic approach is debated controversially, due to the variation in BP responses in individual patients and questionable long-term outcomes64. Activation of both the renin-angiotensin and the sympathetic nervous systems are core mechanisms implicated in renovascular hypertension as demonstrated by increased plasma renin activity and angiotensin II levels, increased firing of efferent sympathetic nerve discharge and whole body NA spillover in these patients65,66. Furthermore, renovascular hypertension represents a potential cause for increased renal afferent signalling, thereby further potentiating efferent sympathetic excitation (Figure1). In this context, catheter-based renal nerve ablation by targeting both efferent and afferent renal nerves appears to be a potential reasonable approach for patients who failed and/or are not suitable for recommended revascularisation. Indeed, RDN has recently been performed successfully in a 68-year-old male non-smoking patient with a long-standing history of hypertension51. Although the patient previously underwent percutaneous transluminal angioplasty for left renal artery stenosis with stent placement, one year following revascularisation his office BP still remained uncontrolled and averaged 174/67 mmHg. Given his intolerance to numerous antihypertensive drug classes, RDN was performed with a total of seven ablation treatments in both arteries. In the left renal artery, four ablation treatments were performed distally to the proximal stent with a safety margin of ~5 mm to avoid interference with energy delivery or heating of the stent (Figure 4). RDN decreased office systolic and diastolic BP post procedure (Figure 5). Baseline resting MSNA was reduced at the three months visit following the RDN procedure (from 30 to 22 bursts/min). No alterations in kidney function or electrolyte disturbances were observed. In addition to the significant BP fall and reduced MSNA, bilateral renal nerve ablation also had a beneficial effect on hypertension-related end-organ damage as evidenced by an improvement of peripheral vascular function including endothelial function (reactive-hyperaemia index - RHI) and ankle brachial index (ABI)51. Whether or not RDN could become an alternative treatment option for renovascular hypertension where other approaches failed needs to be determined.
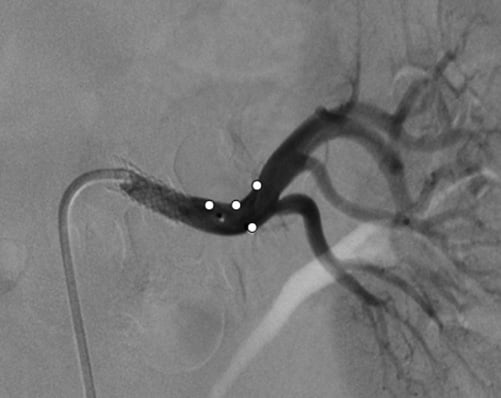
Figure 4. Images of the renal denervation catheter positioned in the lumen of the left renal artery. Ablation sites are marked with white dots. All ablations were delivered distal to the stent. Reproduced with permission from 51.
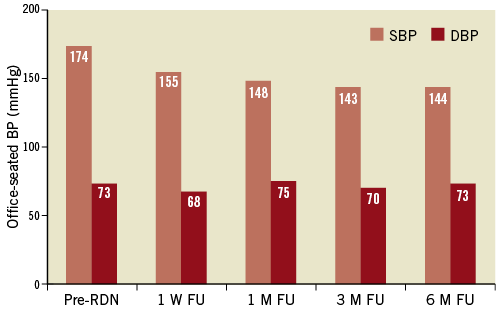
Figure 5. Changes in average office blood pressure at 1 week (W), 1, 3, and 6-month (M) follow-up (FU). Pre-RDN indicates before renal denervation procedure. Reproduced with permission from 51.
Potential limitation for renal denervation in patients with kidney disease
In comparison to patients with RH and normal renal function, patients with CKD especially require more medical attention with regard to potential development of contrast-induced nephropathy. Given the currently available data, RDN is an approach that does not adversely affect kidney function. However, since RDN is an elective procedure, the appropriate pre-hydration and the use of iso-osmolar contrast during the procedure are advisable for patients with impaired renal function. The use of CO2-angiography during the procedure may help to reduce the amount of contrast required49. Subsequently, the longer-term monitoring of renal function and BP control is mandatory following RDN in CKD patients. One of the potential limitations for therapeutic renal nerve ablation in patients with ESRD may be a non-optimal diameter of renal artery anatomy that precludes the catheter position50. Low blood flow through the renal artery may complicate the completion of the ablation treatment periods in a patient with ESRD due to a rapid rise in temperature levels detected by the catheter tip thermistor50. Another concern may be a potentially increased susceptibility to the development of a pseudo-aneurysm at the femoral vascular access site50.
Future directions
The prevalence of CKD and incidence of ESRD attributable to associated comorbidities are estimated to rise further. Despite the efficacy of a wide range of non-pharmacological and pharmacological approaches to lower BP and improve glycaemic control, poorly controlled hypertension and diabetes adversely affect prognosis in renal failure patients. Additional strategies aimed at attaining better BP and glycaemic control in high-risk renal patients may slow the progression of CKD substantially, reduce the overall CV risk and improve patient outcomes. Given the established contribution of sympathetic activation to progressive deterioration of renal function, ablation of both sympathetic efferent and afferent renal nerves appears as an attractive therapeutic alternative. Preliminary clinical findings indicate that catheter-based RDN has a favourable safety profile and improves BP control in CKD and ERSD patients. Current ongoing clinical trials will further define whether catheter-based radiofrequency ablation of sympathetic nerves may translate into better outcomes and reduce risk for major CV events in this high-risk patient population.
Funding
This study was funded in part by grants from the National Health and Research Council of Australia (NHMRC) and the Victorian Government’s Operational Infrastructure Support Program.
Conflict of interest statement
D. Hering was awarded a research fellowship from the Foundation for Polish Science KOLUMB/2010-1. M.P. Schlaich and M.D. Esler are supported by career fellowships from the NHMRC. M.D. Esler and M.P. Schlaich are investigators in studies sponsored by Medtronic and have received honoraria and lecture fees.

