Renal sympathetic denervation (RSD) is a promising treatment option for patients suffering from resistant hypertension1. While various denervation modalities such as radiofrequency (RF) catheter, ultrasound, ethanol injection, and extracorporeal high-intensity focused ultrasound ablation have been developed to deliver energy to the renal sympathetic nervous system, RF-based catheter ablation is most frequently used in current clinical practice2-5. Indeed, there has been a rapid dissemination of this technology with modification of catheter design and application modality of denervation energy2,6-8. To this end, comparative assessment of treatment effects is lacking in both preclinical and clinical studies, and is unlikely to appear on the horizon as it currently stands. Furthermore, reliable comparative assessment of renal denervation devices can only be performed when influencing variables and treatment settings can be controlled appropriately.
In this issue of EuroIntervention, Al Raisi and colleagues developed a novel phantom model to evaluate the spatiotemporal lesion dimensions and dynamics after application of RF energy derived from different denervation catheters9. They compared the Symplicity™ (Medtronic, Minneapolis, MN, USA) with the EnligHTN™ (St. Jude Medical, St. Paul, MN, USA) RF denervation system. The phantom model, which consisted of a hollowed gel block surrounding a thermochromic liquid crystal film, enabled the direct comparison of lesion size in a spatial and temporal dimension. Furthermore, the phantom model allowed testing these outcomes in various settings including alterations in contact area and/or energy, which is ethically and economically difficult to achieve in animal studies. In their results, the Symplicity system achieved overall a larger ablation area as compared to the EnligHTN system. Although this finding cannot be extrapolated to gauge the ablation area expected in man, there seems to be a tendency towards larger lesion dimensions with the Symplicity system. In a simplistic approach, the larger ablation area achieved with the Symplicity system may increase the number of targeted periarterial renal nerves resulting in more effective treatment. However, there are other device-related, design-related, and patient-related factors that are involved in the determination of treatment efficacy when it comes to radiofrequency ablation procedures.
Factors influencing lesion dimension
Since the effects of RF energy depend on multiple factors such as temperature, duration of current application, power, electrode size, and quality of electrode-tissue contact10, the attained ablation area using a phantom model is clearly multifactorial in nature. In this regard, it must be mentioned that the authors adopted different durations (120 seconds for the Symplicity and 90 seconds for the EnligHTN) as recommended by the instructions for use for each device. Furthermore, there are substantial differences in catheter design and number of active electrodes between the Symplicity and the EnligHTN system. For example, the Symplicity catheter has a single electrode system, whereas the EnligHTN system is composed of multiple electrodes (four electrodes) and only one of four electrodes was used for the comparison.
Balancing efficacy and safety
The major finding of a larger ablation area also needs to be interpreted with caution. While the efficacy in targeting periarterial sympathetic nerves may increase, there remains a potential for increased arterial and periarterial tissue damage, which is composed of vein, arteriole and ureter injury. Also, extensive penetration of radiofrequency energy exposes the retroperitoneal organs to thermal injury. There are many local factors associated with the efficacy and safety of renal denervation (RDN) (Figure 1). Local factors such as renal artery anatomy (length, diameter, and accessory/polar artery) and operator experience in RDN procedures obviously affect the efficacy and safety of RDN (renal artery damage)11, whereas periarterial nerve distribution12,13 or the presence of heat reservoir (lymph nodes or vein) predominantly affect the efficacy of RDN (nerve injury).
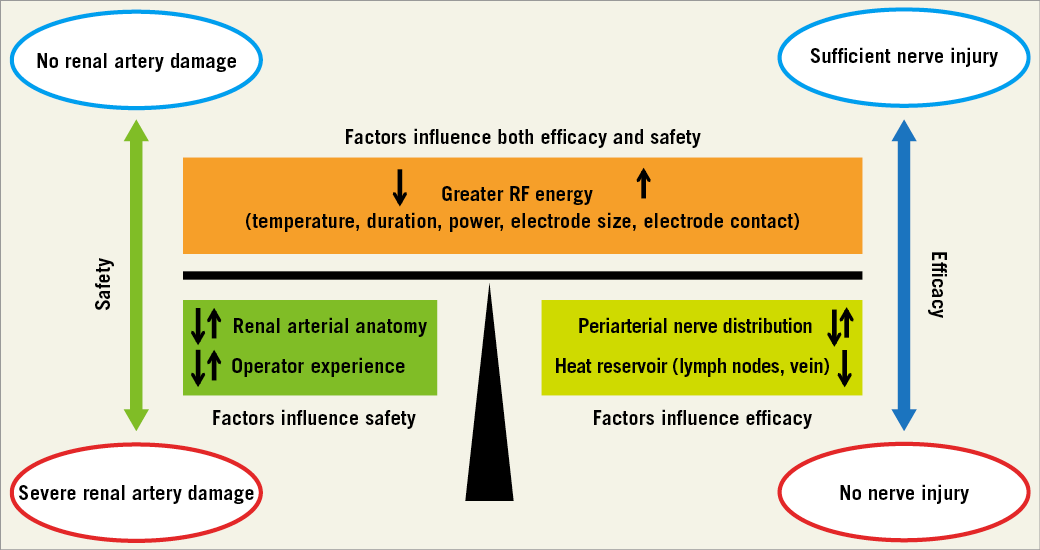
Figure 1. Local factors associated with the efficacy and safety of renal denervation.
The integrity of the renal artery can be confirmed by optical coherence tomography more precisely as compared to renal angiography14. Although periarterial nerves cannot be visualised in the clinical application of this technology, the knowledge of human renal nerve anatomy provides valuable guidance during RDN procedures12 as it helps to determine treatment points, duration and durability of effects.
Clinical implications
In the light of the recent clinical development of RDN therapy where the initial euphoria was suddenly dampened by a failed randomised pivotal trial in the USA comparing RDN therapy to sham treatment in patients with resistant arterial hypertension15, realignment of research activities towards a better understanding of molecular and preclinical effects of this novel technology seems to be mandatory to conquer an important hurdle to clinical success. Experimental studies such as the current one certainly contribute to our understanding of RDN effects as individual factors pertaining to both efficacy and safety can be investigated in the absence of confounding covariates, which are impossible to study in an in vivo environment. However, an important gap between experimental/preclinical and clinical studies in this field refers to the absence of appropriate surrogate parameters of efficacy, which makes a direct translation of experimental/preclinical findings to clinical practice impossible. In order to close this gap, further research should be focused on functional aspects of RDN therapy which show similarities between preclinical animal models and man. In addition, animal models of arterial hypertension may help boost our understanding of RDN-associated effects and help identify patients who may benefit most from this novel technology.
Conflict of interest statement
K. Sakakura has received speaking honorarium from Abbott Vascular, Boston Scientific, and Medtronic CardioVascular. M. Joner is a consultant for Biotronik and Cardionovum, and has received speaking honorarium from Abbott Vascular, Biotronik, Cordis J&J, Medtronic, and St. Jude.

