
Abstract
Aims: This study aimed to evaluate the feasibility and safety of the left distal transradial approach (ldTRA) as a default route for coronary angiography (CAG) and percutaneous coronary intervention (PCI).
Methods and results: Between October 2017 and January 2018, 200 consecutive patients were enrolled in a single centre. The left distal radial artery was punctured with a 20-gauge venipuncture catheter needle by three expert left radial approach operators. The success rates of arterial puncture, CAG, and PCI were 95.5% (191/200), 100% (187/187), and 98.9% (86/87), respectively. Four patients scheduled for staged PCI skipped the routine diagnostic CAG. Puncture time and fluoroscopic time were 3.0±2.8 minutes and 11.3±18.4 minutes, respectively. Haemostasis time was 151.8±39.9 minutes. A total of 15 (7.9%) puncture site complications occurred, including 14 (7.4%) minor haematomas and one (0.5%) arterial dissection, in which the artery was patent at one-month follow-up. Two patients complained of left thumb numbness at one-month follow-up. No distal radial artery occlusion, perforation, pseudoaneurysm, or arteriovenous fistula occurred.
Conclusions: The success and complication rates of ldTRA support the feasibility and safety of this procedure. Larger randomised comparison studies are needed to support this preliminary evidence.
Abbreviations
BARC: Bleeding Academic Research Consortium
CAG: coronary angiography
DRA: distal radial approach
Fr: French
ldTRA: left distal transradial approach
NSTEMI: non-ST-segment elevation myocardial infarction
PCI: percutaneous coronary intervention
RA: radial approach
STEMI: ST-segment elevation myocardial infarction
VAS: visual analogue scale
Introduction
The left radial approach (RA) has several advantages compared to the right RA in terms of shorter fluoroscopic time, lesser contrast use, and a lower incidence of procedure-related stroke with similar access-site complications1-4. However, most radial operators still prefer the right RA because of operator convenience.
Recently, the left distal transradial approach (ldTRA) has been proposed as an alternative to the right RA5. Kiemeneij reported early experience with ldTRA in 70 selected patients with a good distal radial pulse. This approach includes the puncture of the distal portion of the radial artery via the anatomical snuffbox. However, there is a lack of evidence for the routine use of ldTRA in terms of puncture success, procedural success, procedure-related complications, and learning curve. Therefore, the aim of this study was to evaluate the feasibility and safety of the ldTRA as a default route for coronary angiography (CAG) and percutaneous coronary intervention (PCI).
Methods
STUDY DESIGN AND POPULATION
The left distal transradial approach (LeDRA) trial was a prospective, observational registry (ClinicalTrials.gov identifier: NCT03292367). Between October 2017 and January 2018, 200 patients with a palpable left distal radial artery planned for CAG were consecutively enrolled in a single centre (Figure 1). Patients without a palpable left distal radial artery, with a positive modified Allen test suspicious of ulnar artery occlusion, or considered unsuitable by the investigator, were excluded. This study was approved by our Institutional Review Board, and all patients provided written informed consent to participate in this study. During three months before study enrolment, three experienced radial operators (more than eight years’ experience) discussed the puncture technique, type of puncture needle, hand position, and haemostasis method in an early experience on 30 cases. After the set-up of the overall procedural protocol, patients were consecutively enrolled.
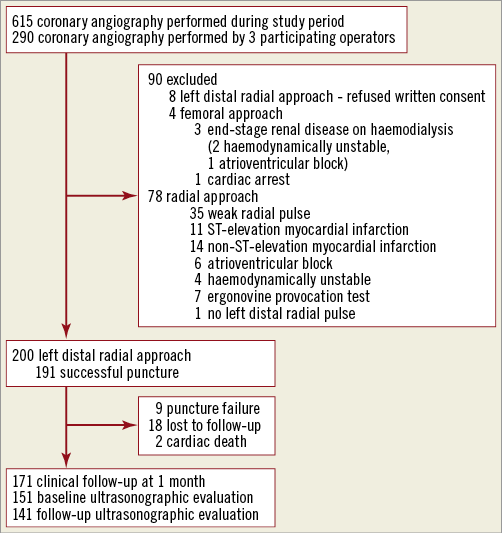
Figure 1. Study flow diagram.
PREPARATION
Topical EMLA (Laboratoires Astra-france, Nanterre, France) anaesthetic cream (lidocaine 2.5% and prilocaine 2.5%) was applied over the skin area of both radial arteries and distal radial arteries at least 30 minutes before the puncture in order to reduce pain6. Then, a transparent film (3M™ Tegaderm™; 3M, St. Paul, MN, USA) was applied to each site (Figure 2). The left hand in the prone position was placed either on the left groin or beside the left hip according to operator preference. After removing the transparent film and anaesthetic cream, the left hand was disinfected over the anatomical snuffbox, palm, and medial side of the wrist with povidone-iodine and alcohol gauze. The patient was covered with a sterile drape with four holes (two radial and two femoral holes). Both femoral preparations were carried out simultaneously in the case of high-risk patients, such as those with acute myocardial infarction and haemodynamic instability.
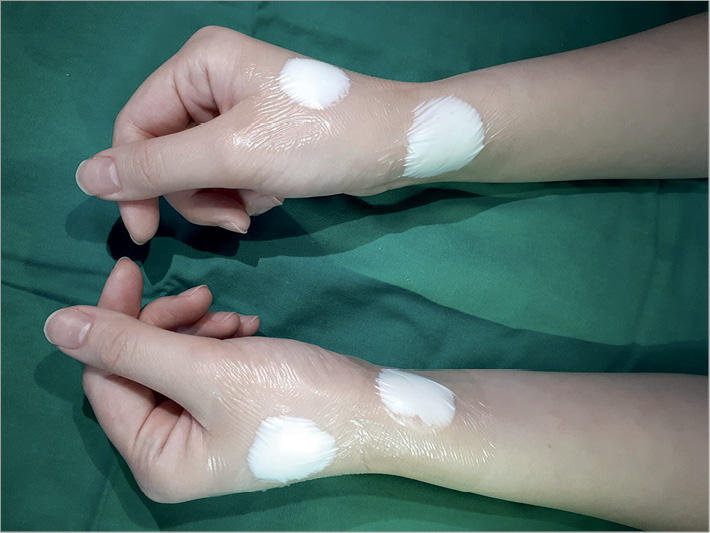
Figure 2. Application of topical EMLA anaesthetic cream.
PUNCTURE OF THE LEFT DISTAL RADIAL ARTERY
The operator evaluated the pulse, tortuosity, and size of the left distal radial artery and radial artery before the puncture. A small skin incision, at the operator’s discretion, was made before the puncture and insertion of the introducer sheath. After subcutaneous injection of lidocaine, the artery around the bony surface area was punctured with a 20-gauge venipuncture catheter needle (Moving image 1-Moving image 3). The angle of the needle was maintained at less than 30 degrees in order to facilitate the puncture of the anterior arterial wall and to minimise periosteal pain from the needle tip. The artery was fixed with the fingers of the other hand to minimise its movement. After successful puncture, the metallic needle was removed and a flexible, straight plastic 0.025” mini guidewire was inserted through the venipuncture catheter. If the mini guidewire failed to pass through, a 0.014” guidewire was advanced first, followed by advancing the venipuncture catheter further, and then exchanged for a 0.025” guidewire. After the venipuncture catheter had been placed into the artery, nitroglycerine was administered, and angiography of the left distal radial artery and radial artery was performed to check the vessel size, tortuosity, and puncture-related complications (Moving image 4, Moving image 5). Then, a Radifocus® introducer sheath (Terumo Corp., Tokyo, Japan) was inserted.
PROCEDURE
The side tube of the introducer sheath was connected to an arterial pressure monitor. After confirmation of the arterial waveform on the monitor, a bolus of 5,000 units of heparin was injected into the radial artery. Before crossing the diagnostic or guiding catheter, the left elbow was straightened to avoid kinking resistance to the catheter. Diagnostic CAG and PCI were performed in the usual manner. An additional 5,000 units of heparin were administered before PCI. If the size of the distal radial artery was small (outer diameter less than 2.3 mm)7, a sheathless 6.5 Fr guiding catheter (Asahi Intecc, Aichi, Japan) was used.
HAEMOSTASIS
After finishing the overall procedure, the introducer sheath was pulled out about 2-3 cm from the puncture site. Then, a haemostasis pad (2×2 cm) was placed at the puncture site. Sterile 4”×4” gauze was wrapped and compressed using 3M™ Transpore™ (3M) adhesive tape with a firm pressure (Moving image 6). During haemostasis, about 2-3 cm of empty space was kept in order to maintain venous drainage and avoid resultant hand oedema. In patients with thin or fragile skin, the puncture site was wrapped with 3M™ Coban™ (3M) self-adherent bandage under the haemostasis pad and 4”×4” wrapped gauze (Figure 3). The compression site was checked by operators after two to three hours of haemostasis. If the routine check-up revealed no bleeding or haematoma, compression was released. However, if a bleeding at the puncture site or swelling with minor haematoma was detected, an additional 30 minutes to one hour of compression was maintained.
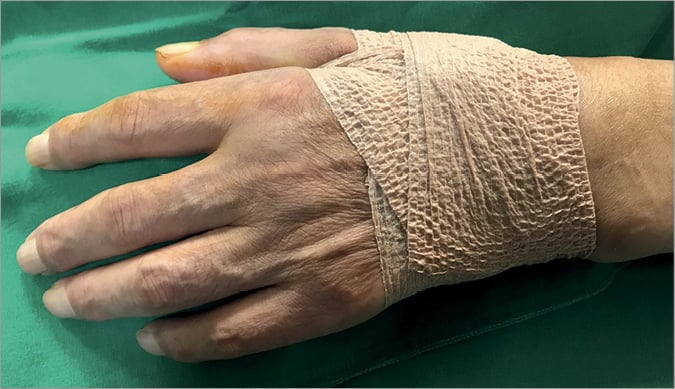
Figure 3. Compression using 3M™ Coban™ self-adherent bandage.
ASSESSMENT OF DIAMETER BY ULTRASONOGRAPHY
When feasible, the size of both radial and distal radial artery was assessed before puncture by ultrasonography. The outer diameters of the artery were measured by perpendicular angle, and the average value was recorded. At one-month follow-up, arterial occlusion and puncture-related complications were assessed via Doppler ultrasonography.
STUDY ENDPOINTS
The primary endpoints were the success rate of CAG and PCI. Secondary endpoints included the success rate of puncture of the left distal radial artery, complications at the puncture site, puncture time, procedure time, fluoroscopic time, fluoroscopic dose, contrast volume, haemostasis time, diameter of the left distal radial artery by ultrasonography, and questionnaire for pain and satisfaction. The Bleeding Academic Research Consortium (BARC) classification was used to define bleeding complications. Haematoma at the puncture site was graded into three groups (<2 cm, 2-5 cm, >5 cm). Bleeding events were adjudicated by three participating investigators.
QUESTIONNAIRE
A questionnaire for the ldTRA was provided if possible. The visual analogue scale (VAS) was used to assess the severity of pain and satisfaction8. Patients were requested to express the pain and satisfaction as a number from 0 to 10. A score of 7 or more for pain was defined as severe. A score of less than 4 for satisfaction was defined as unsatisfactory.
STATISTICAL ANALYSIS
For continuous variables, data are expressed as mean±standard deviation or median (interquartile range). For categorical variables, data are expressed as counts and percentages. To evaluate the learning curve, patients were divided into four groups of 50 cases over time. A one-way analysis of variance was used to compare the mean puncture time and total procedure time among the four groups. Statistical analysis was performed with SPSS software, Version 22.0 (IBM Corporation, Armonk, NY, USA). P-values <0.05 were considered statistically significant.
Results
PATIENT POPULATION
Table 1 summarises the baseline characteristics of all included patients. Fifteen (7.5%) patients had a medical history of chronic kidney disease and three (1.5%) patients were on dialysis. A total of 37 (18.5%) patients had creatinine clearance <60 mL/min. Seventeen (8.5%) patients had ST-segment elevation myocardial infarction and 45 (22.5%) patients had non-ST-segment elevation myocardial infarction. There were two cases of cardiac death not related to the procedure during hospitalisation.
PROCEDURAL CHARACTERISTICS
Table 2 shows procedural results for the patients with successful puncture of the left distal radial artery. CAG was performed in 187/191 (97.9%) patients. Four patients scheduled for staged PCI skipped the routine diagnostic CAG. The introducer sheaths used for diagnostic CAG were 87 (45.5%) 4 Fr size, 41 (21.5%) 5 Fr size, 62 (32.5%) 6 Fr size and one (0.5%) 7 Fr size. A single diagnostic catheter was used in 102 (53.4%) patients who underwent CAG. Eighty-seven (45.5%) patients needed PCI. Ad hoc PCI was performed in 78 (89.7%) patients, including 28 (32.2%) with bifurcation lesions, 9 (10.3%) with in-stent restenosis, and 8 (9.8%) with chronic total occlusion. Sixty-two (71.3%) patients were treated using a 6 Fr guiding catheter. A sheathless 6.5 Fr guiding catheter was used in 19 (21.8%) patients with a small distal radial artery.

PRIMARY AND SECONDARY ENDPOINTS
The success rates of CAG and PCI were 100% (187/187) and 98.9% (86/87), respectively (Table 3). One case of PCI failure was diagnosed with chronic total occlusion of the left anterior descending artery, and the procedure was stopped because the guidewire failed to pass into the true lumen. The success rate of distal radial artery puncture was 95.5% (191/200). Crossover sites in seven (3.5%) patients were in the left radial artery and in two (1%) patients were in the right distal radial artery. Among 191 cases of the distal radial approach, puncture time was 3.0±2.8 minutes and haemostasis time was 151.8±39.9 minutes. The questionnaire for pain and satisfaction was completed by 182 of 191 patients (95.3%). Three patients were unable to understand the questionnaire, five patients refused and one patient died before the questionnaire. The mean pain score was 2.6±2.4 and the mean satisfaction score was 9.0±1.5. Seventeen (9.3%) patients complained of severe pain (≥7 score). Three (1.6%) patients answered unsatisfactory (<4 score) for the distal radial approach.

ACCESS-SITE COMPLICATIONS
No major bleeding, defined as BARC bleeding type 2, 3, or 5, occurred during the study period (Table 4). Minor haematoma occurred in 14 (7.4%) patients. There was no distal radial artery occlusion, perforation, pseudoaneurysm or arteriovenous fistula. One patient had a puncture-related arterial dissection, but the vessel was patent at follow-up ultrasonographic evaluation. Follow-up ultrasonographic evaluation was performed in 141 (73.8%) patients. Two (1.4%) patients complained of numbness around the puncture site at one-month ultrasonographic follow-up.

ULTRASONOGRAPHIC EVALUATION
The diameter of both radial and distal radial artery was assessed in 151 patients before CAG (Table 5). The diameter of the left distal radial artery was larger than that of the introducer sheath in 90/151 (59.6%) patients who underwent CAG and in 29/63 (46%) patients who underwent PCI. Figure 4 shows the cumulative percent of measured diameter of the left distal radial artery (Figure 4A) and radial artery (Figure 4B). According to this measurement, the left distal radial artery was considered suitable for a 5 Fr introducer sheath (outer diameter 2.3 mm) only in 57.6% of patients and a 6 Fr introducer sheath (outer diameter 2.7 mm) only in 35.1% of patients. On the other hand, 5 Fr and 6 Fr introducer sheaths were considered compatible to the left radial artery in 94% and 80.1% of patients, respectively.
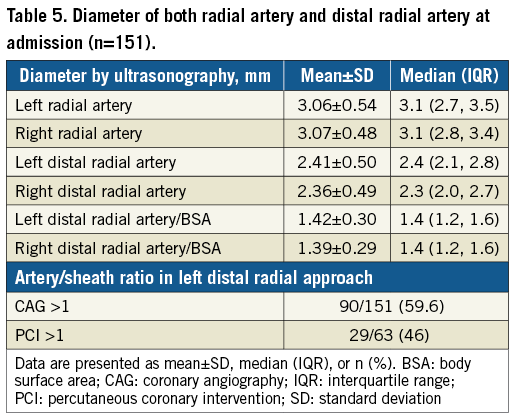
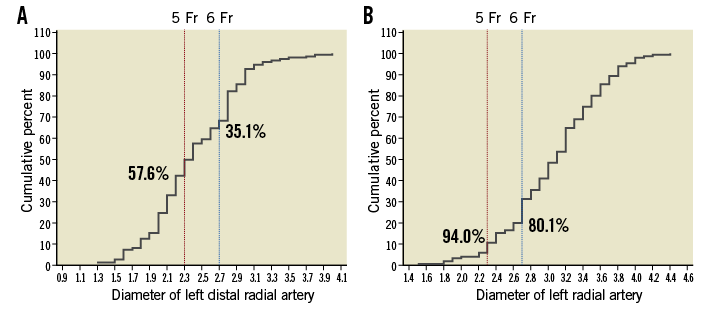
Figure 4. Cumulative percent of artery diameter measured by ultrasonography. A) Left distal radial artery. B) Left radial artery. % indicates compatible patients for 5 or 6 Fr introducer sheath.
LEARNING CURVES FOR PUNCTURE TIME AND TOTAL PROCEDURE TIME BY EXPERIENCE
Patients were divided into four groups of 50 cases over time. The first 30 patients prior to the study were not included in the learning curve analysis. Puncture time was significantly faster in the last quarter (p<0.009) (Figure 5A). The total procedure time decreased significantly over time (p=0.031) (Figure 5B).

Figure 5. Learning curves. A) Puncture time. B) Total procedure time.
Discussion
This was the first Korean prospective observational registry to evaluate whether ldTRA can be selected as a default route in performing CAG and PCI. We demonstrated that the success and complication rates of ldTRA support the feasibility and safety of this new approach.
Successful puncture of the distal radial artery was the key factor for this study. We tried to puncture the distal radial artery first, if the operator could feel a good distal radial pulse. We reviewed and discussed the method of Kiemeneij before this trial. Then, we set up an overall procedural protocol for the standardisation of this new technique among three operators. The acceptable success rate of puncture may have resulted from the sufficient acquisition of technical skills and the prepared protocol. The high-volume radial PCI centre (average 800 PCI procedures/year) using the left RA could be another reason.
Selecting a proper introducer sheath size may be important, because a larger size of sheath and a smaller artery diameter are well-known predictors of arterial occlusion9-12. In this registry, the procedure was performed with a larger sheath than the artery diameter only in 59.6% of patients who underwent CAG and in 46% of patients who underwent PCI. However, no distal radial occlusion was observed by Doppler ultrasonography at one-month follow-up. Although the patency was not checked in 26.2% of patients, this result suggests that ldTRA could be a promising route for reducing the rate of arterial occlusion. Whether the size discrepancy between the distal radial artery and introducer sheath affects distal radial arterial occlusion should be confirmed in future studies by using Doppler ultrasonography.
This study demonstrated very low complication rates. No major haematoma occurred and 7.4% of patients had a minor haematoma. Thirteen (6.8%) patients needed recompression. Improper position of compression, concomitant use of potent dual antiplatelet agents with anticoagulants, old age, fragile skin, and multiple puncture attempts may be the reasons for minor haematoma. A standardised protocol of compression time and a dedicated compression device may reduce the rate of potential bleeding complications. Additionally, overcoming the learning curve for puncture is important to minimise multiple needle injury. There were two cases of neuropathy at one-month follow-up. The neuropathy may have resulted from needle injury or long duration of compression time.
Although several studies and meta-analyses have been performed to reveal procedural differences and clinical outcomes between the left RA and right RA, there is no conclusive evidence for the left RA over the right RA for coronary procedures. A meta-analysis including 22 clinical trials with 10,287 patients demonstrated that both radial approaches had a similar success rate in coronary procedures1. Fluoroscopic time and contrast use were significantly lower in the left RA. The incidence of subclavian tortuosity was higher in the right RA (12.96%) compared to the left RA (2.78%) (p=0.028). Therefore, the procedural differences may have originated from the anatomical variation rather than the approach site. The recently updated meta-analysis also reported the significant reduction of fluoroscopic time and contrast use in the left RA2. There were no differences in total procedure time and crossover rate between both radial approaches. In the Korean transradial coronary intervention registry, both radial approaches had similar 12-month clinical outcomes in patients undergoing PCI3. The prospective national registry from the British Cardiovascular Intervention Society (BCIS) including 342,806 cases illustrated that the left RA and right RA had no significant difference in terms of clinical adverse events regarding in-hospital mortality, 30-day mortality, major adverse cardiovascular events or major bleeding4. Interestingly, the procedure-related stroke rate was reduced in the left RA according to propensity-matched analysis. Therefore, the use of ldTRA could reduce procedure-related stroke where operators are proficient in the right RA. A randomised comparison study is needed to uncover the potential benefits and complications of ldTRA.
This study also evaluated the learning curve for puncture time. Puncture time stabilised after approximately 150 cases. Small artery diameter, weak pulse, stiff artery, and old age seemed to be the reasons for delayed puncture, although no data could be obtained. In addition, the puncture of the distal radial artery needs more skill than the radial artery puncture. Because the distal radial artery is movable, the fixation of the artery with the other hand is helpful to increase the puncture success rate. Furthermore, the puncture angle may be important. Maintaining this angle at less than 30 degrees can increase the chance of the puncture of the anterior wall of the artery and reduce the periosteal injury.
The proven benefit of ldTRA compared to the RA or femoral approach is uncertain. However, the absence of radial injury can have a potential benefit in patients requiring arteriovenous fistula and in patients where the radial artery is used as a conduit for coronary artery bypass graft. The distal radial approach (DRA) can also reduce the incidence of potential bleeding complications in patients with a heavily calcified and diseased femoral artery. Whether the DRA can reduce arterial occlusion and bleeding complications requires further investigation.
Limitations
This study has several limitations. First, this study was conducted in a single centre. The feasibility and safety of the results obtained should be confirmed by a large, multicentre study. Second, a relatively small number of patients was enrolled without a control group in this study. Based on this study’s results, a comparison study between the DRA and RA should be performed. Third, eighteen (9%) patients were lost to follow-up, which may be a great limitation considering the low number of patients included. Finally, the follow-up image study was not performed in all patients, which could have led to an underestimation of procedure-related complications.
Conclusions
The success and complication rates of ldTRA support the feasibility and safety of this procedure. Larger randomised comparison studies are needed to support this preliminary evidence.
| Impact on daily practice Although the left radial approach has shown several advantages of shorter fluoroscopic time, lesser contrast use, lower incidence of procedure-related stroke and similar complication rates compared to the right radial approach, the default route for most radial operators is the right radial artery because of operator preference. The left distal transradial approach could be considered a feasible and safe alternative to the right radial approach based on the result of this prospective observational study. |
Funding
Samjin Pharmaceutical Co., Ltd., and the Gangwon Chapter of the Korean Society of Cardiology sponsored the LeDRA trial.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Anterior puncture of the distal radial artery at the right side.
Moving image 2. Anterior puncture of the distal radial artery at the left side.
Moving image 3. Posterior puncture of the distal radial artery at the left side.
Moving image 4. Distal radial artery angiography.
Moving image 5. Radial artery angiography.
Moving image 6. Manual compression using 3M™ Transpore™ adhesive tape.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Anterior puncture of the distal radial artery at the right side.
Moving image 2. Anterior puncture of the distal radial artery at the left side.
Moving image 3. Posterior puncture of the distal radial artery at the left side.
Moving image 4. Distal radial artery angiography.
Moving image 5. Radial artery angiography.
Moving image 6. Manual compression using 3M™ Transpore™ adhesive tape.

