
Abstract
Aims: The aim of this technical report is to demonstrate the feasibility of the left distal transradial approach for patients in whom left radial access is preferred over right radial access for coronary angiography and interventions. This procedure is more convenient for the operator. For the right-handed patient, the left radial access is more convenient because of the free use of the right hand after the procedure. In addition, this technique reduces the chance of radial artery occlusion at the site of the distal forearm.
Methods and results: Coronary access via the left distal radial artery at the anatomical snuffbox allows comfortable positioning of the dorsal side of the patient’s left hand near the right groin. The operator can puncture the artery and perform the coronary cannulation at a safe distance from the radiation source and without the need to bend over the patient. This technique will be described in detail. Procedural and clinical results in the first 70 patients are described. Out of 118 consecutive patients assigned to the author’s operation programme, 70 patients were considered suitable for left distal radial access. There were eight procedural failures, requiring crossover to traditional right radial or left radial approach. All other procedures were successful, without major discomfort for the patient and operator. No radial artery occlusions at the site of the forearm were encountered.
Conclusions: Left distal transradial coronary access via the anatomical snuffbox, as default technique for patients who need or prefer left radial access over right radial access, deserves further exploration.
Abbreviations
CAG: coronary angiography
Fr: French
G: gauge
ldRA: left distal radial artery
ldTRA: left distal transradial coronary angiography
ldTRI: left distal transradial coronary intervention
LIMA: left internal mammary artery
NSTEMI: non-ST-elevation myocardial infarction
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TRA: transradial angiography
TRI: transradial intervention
VAS: visual analogue scale
Introduction
Since the early publications from 1993 onwards, the transradial approach can now be considered as the default technique for coronary access1-4. The major advantages are increased safety, because of reduction of major bleeding complications, and increased patient comfort, because of immediate post-procedural mobilisation5,6.
Most operators prefer the right radial approach. The main reason is the working position of the operator on the right side of the patient.
However, frequently the operator needs to cross over to the left radial approach. The most common reasons to use the left radial artery instead of the right radial artery are: right radial occlusion, underdeveloped right radial artery, extreme right radial tortuosity, sclerosis or calcifications, arteria lusoria, previous right radial failure, presence of an arteriovenous shunt in the right arm, past or future use of the right radial artery as free arterial graft, post-CABG patients requiring LIMA angiography, and patient preference. Without any doubt, the left radial access is more convenient for right-handed patients because of temporary post-procedural disability by the haemostasis process.
In terms of feasibility and outcome, left and right transradial approaches are similar7. However, the left radial access is cumbersome for the operator, especially in obese patients. The arm in the volar position limits flexion of the forearm towards the operator. The operator therefore needs to bend over the patient, which is ergonomically very unpleasant. In addition, the operator is exposed to higher radiation doses because of closer proximity to the radiation source and due to radiation scatter from the patient’s body.
A simple solution is to access the left distal radial artery from the anatomical snuffbox (radial fossa, fovea radialis) on the dorsal side of the hand. The anatomical snuffbox is a hollow space on the radial side of the wrist when the thumb is extended; it is bounded by the tendon of the extensor pollicis longus posteriorly and of the tendons of the extensor pollicis brevis and abductor pollicis longus anteriorly. The radial artery crosses the floor that is formed by the scaphoid and the trapezium bones8. Distal radial artery access from the radial fossa was described for the first time by Babunashvili et al in order to open occluded ipsilateral radial arteries in a retrograde fashion9. If well developed, this artery can be used as an entry site for 4, 5 and 6 Fr sheaths and catheters10. The left arm can be brought comfortably towards the right side of the patient, allowing a natural working position for the operator.
Another important feature of this technique is a puncture proximal from the pollicis brevis artery and distal from the branch supplying the superficial palmar arch. An occlusion at this site maintains antegrade flow through the superficial palmar arch. This reduces the risk of retrograde thrombus formation in the proximal radial artery located in the forearm, a frequent finding in patients who develop a forearm radial artery occlusion due to puncture trauma or haemostasis trauma at the traditional radial puncture site. Flow to the thumb will still be maintained via the superficial palmar arch, preventing ischaemia and hand disability.
Methods
The presence of a well-developed distal radial artery in the radial fossa is detected by manual palpation. In order to avoid puncture of one of the terminal branches, the puncture is performed at the most proximal part of the anatomical snuffbox.
PATIENT POSITION AND PREPARATION
The left upper am is placed comfortably on a cushion on the left side of the patient. The left hand is bent over towards the patient’s right groin (Figure 1). After disinfection, the patient is covered with a sterile drape containing four holes (two radial, two femoral). The left femoral hole is placed over the dorsal side of the patient’s left hand (Figure 2). The operator takes up a position near the patient’s head for subcutaneous injection of 3-5 cc xylocaine filling the radial fossa, puncture and sheath insertion (Figure 3). To bring the artery to the surface of the fossa, the patient is asked to grasp his thumb under the other four fingers, with the hand slightly abducted (Figure 4). The artery is punctured, preferably with a 21 gauge (G) open needle, under an angle of 30-45 degrees and from lateral to medial. The needle is directed to the point of strongest pulse, proximal in the anatomical snuffbox (Figure 5). A through-and-through puncture is not recommended, since the needle will touch the periostium of the scaphoid or trapezium bones, which can be painful. After successful puncture, a flexible, soft, J-shaped 0.21” metallic wire is inserted. In order to prevent damage to the tip of the introducer and sheath, which might damage the artery, a small skin incision is made (Figure 6), followed by introducing the 4, 5 or 6 Fr sheath (Figure 7). After administration of a spasmolytic cocktail containing 200 mcg of nitroglycerine and 5 mg of verapamil and of a weight-adjusted dose of heparin, the operator can take up a position at the level of the patient’s knees (Figure 8) to manipulate the 0.35” wire, the catheters and the intracoronary devices. If resistance is encountered with the wire at the level of the flexed elbow, a hydrophilic wire will cross.
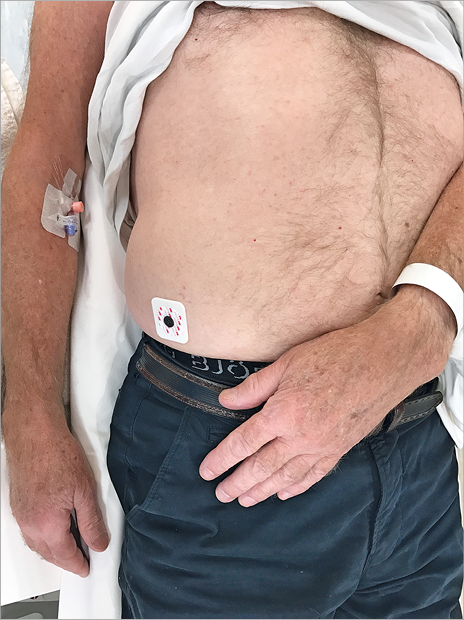
Figure 1. The left hand of the patient is placed on the right groin, dorsal side up.
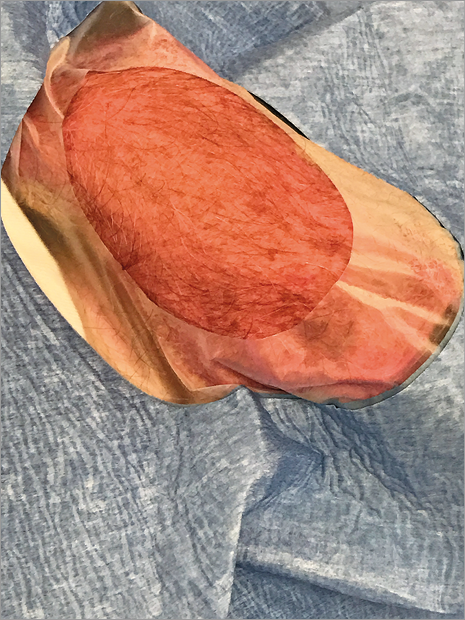
Figure 2. Radial fossa prepared for access.
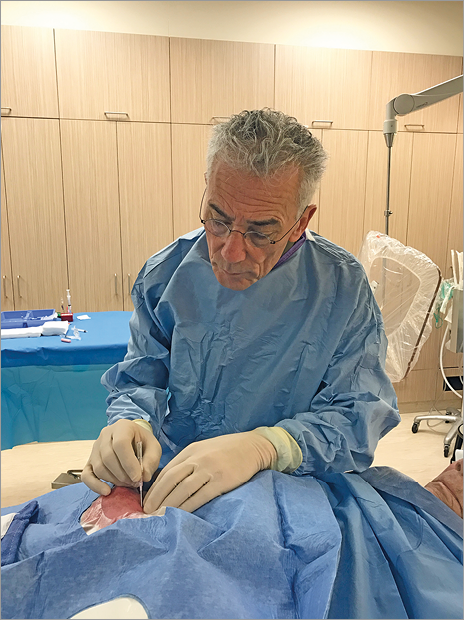
Figure 3. Operator position for anaesthesia, puncture and sheath insertion.
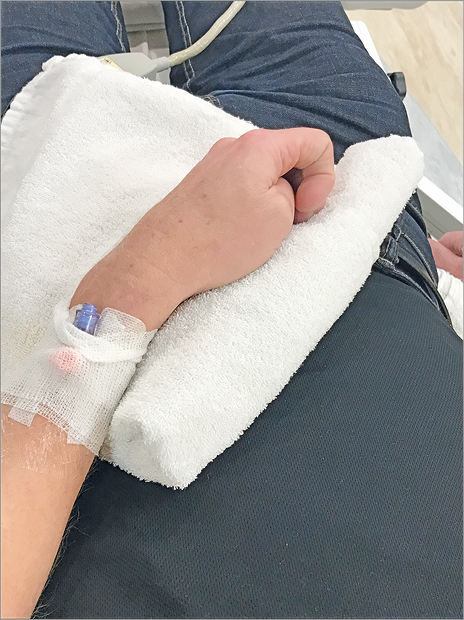
Figure 4. The artery is brought to the surface of the anatomical snuffbox.
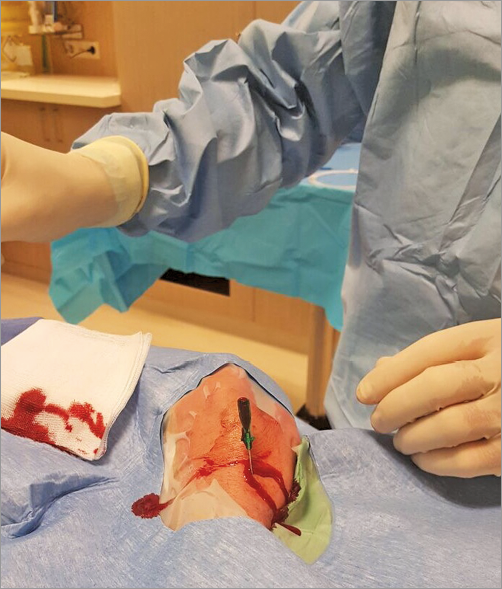
Figure 5. The needle is directed to the point of strongest pulse, proximal in the anatomical snuffbox.
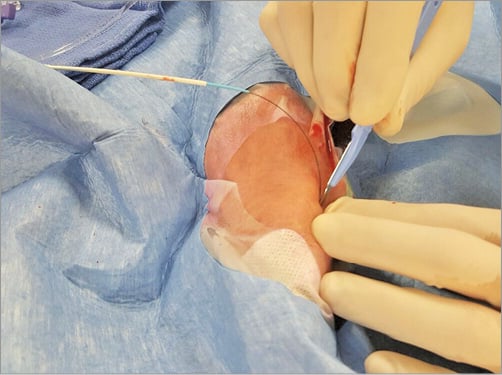
Figure 6. A small skin incision is made before introducing the sheath.
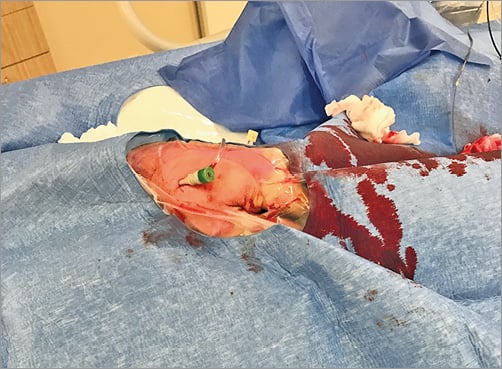
Figure 7. 6 Fr sheath in situ.
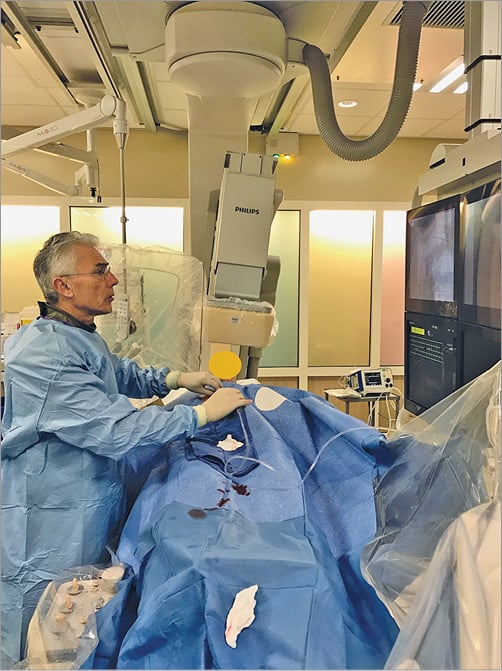
Figure 8. The operator takes up position at the knee level of the patient.
HAEMOSTASIS AND CONTROL
After the procedure, the sheath is pulled out for 3 cm, after which a SafeGuard® (Merit Medical Systems, South Jordan, UT, USA) haemostasis band is placed over the puncture site. At inflation of 3 ml of air into the air compartment, the sheath is pulled out completely, followed by extra injection of 2 ml of air. This band is left in situ for two, or maximally three hours (Figure 9), after which deflation of air is started and completed within half an hour. Alternatively, a small pile of gauze is placed over the puncture site during sheath removal, followed by application of a semi-elastic bandage, which is left in situ for two to three hours (Figure 10). Before discharge, the presence of a radial pulse at the distal forearm and in the anatomical snuffbox is checked by manual palpation and Doppler ultrasound.
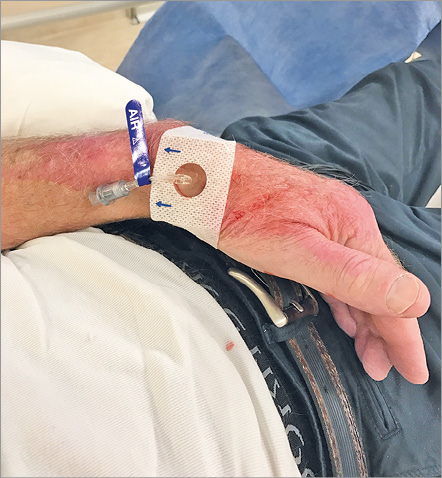
Figure 9. SafeGuard (Merit Medical) is left in situ for two to three hours.
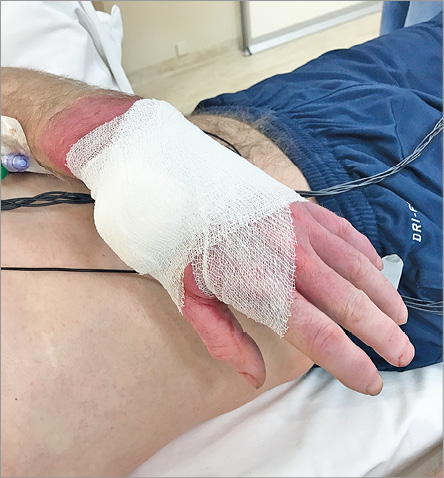
Figure 10. Manual compression bandage is left in situ for three hours.
Results
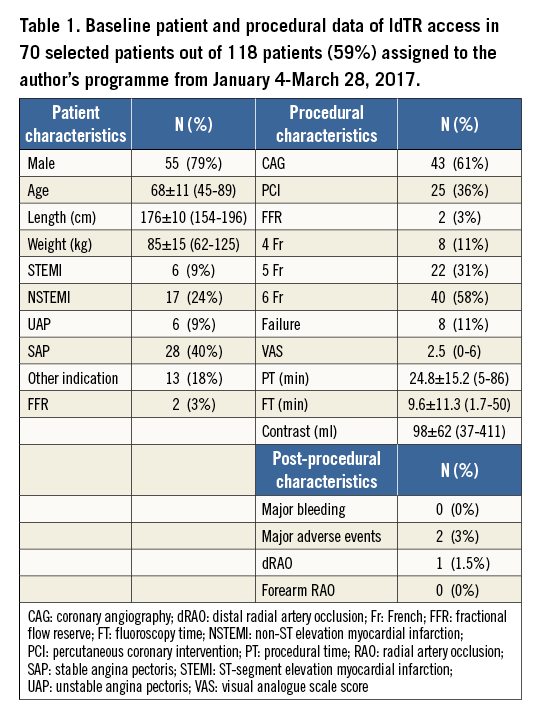
From January to March 2017, 70 out of 118 patients (59%), assigned to the author’s operating programme, underwent left distal transradial (ldTR) access. The main reasons not to perform ldTR access in the remaining 48 patients (41%) were weak or absent pulse in the anatomical snuffbox (n=27; 23%), logistical reasons (n=7; 6%), presence of an indwelling venous cannula in or near the snuffbox (n=6; 5%), left-handedness (n=4; 3.5%), and patient preference for several reasons (n=4; 3.5%).
Patient and procedural characteristics are summarised in Table 1. Of the 70 patients who underwent ldTR coronary access, eight (11%) had a failed attempt. In four patients the puncture failed, while in four patients the puncture was successful, but the wire could not be advanced towards the forearm part of the radial artery. In most patients 6 Fr sheaths were used (n=40; 58%), followed by 5 Fr sheaths (n=22; 31%) and 4 Fr sheaths (n=8, 11%). One patient reported relevant discomfort during injection of an intra-arterial cocktail and heparin (visual analogue scale [VAS] 6). No other patients had relevant discomfort at puncture, sheath insertion, catheter manipulation and haemostasis (VAS 2.5). On average, the amount of contrast used was 98 ml. Overall mean fluoroscopy time was 9.6 minutes. Procedural time was 24.8 minutes, ranging from five to 86 minutes. This long procedure was due to cannulation failure of an aberrant right coronary artery in a patient with an occluded right radial artery. Crossover to the right femoral approach did not result in cannulation success either. Ten different catheter shapes were used. This patient developed temporary dysphasia the next day. In another patient, a dissection just proximal to the origin of the left subclavian artery was visible after an attempt to advance a 0.35” wire over a tortuous segment. The dissection extended towards the aortic root and the patient was operated. One patient died of in-hospital cardiac arrest two days after PCI for an ambulant anterior infarction and poor left ventricular function. Other patients had an uneventful clinical follow-up. Haemostasis was obtained within three hours in all patients. No major bleeding, requiring prolonged hospital stay, surgery or transfusion, was encountered. One patient developed an ecchymosis of the hand, and one patient had a conservatively managed forearm bleeding, probably because of unnoticed wire perforation of a side branch from the proximal radial artery. Follow-up assessment at discharge was obtained in 50 of 62 patients (81%) with a successful ldTR procedure. All radial arteries were open at the “conventional” forearm site. One patient had an occluded distal radial artery. No patient complained of local numbness or dysfunction of the hand.
Discussion
This early experience demonstrates the feasibility of the left radial approach via the distal radial artery located at the anatomical snuffbox. The procedures were performed with 4, 5 and 6 Fr catheters and in stable and acute patients, for simple and complex lesions. On average the VAS score was low. The advantages of this approach are several. First of all, the right-handed patient is still free to use the right arm unrestrictedly after the procedure. The arm position during the intervention is comfortable for the patient, who does not have to expose the palmar side of the arm while flexing the upper arm towards the operator. No equipment or investments are necessary to support the patient’s left arm. The operator can work as usual from the right side of the patient and does not need to bend over the patient to reach for the left radial artery, which is very cumbersome, especially if the patient is obese and if the operator is not tall. In terms of ergonomics, ldTR access is similar to right radial procedures. The operator can work at a safe distance from the radiation source. An additional and potentially important advantage is reduction of the risk of radial artery occlusion at the site of “conventional” point of entry in the distal forearm. There is no puncture trauma, no vessel wall damage by sheath introduction and no trauma by prolonged haemostatic occlusion. Since antegrade flow through the superficial palmar arch is still maintained, the radial artery will not thrombose in case of occlusion of the radial artery in the snuffbox. This is relevant for patients requiring multiple radial artery procedures or requiring coronary bypass surgery with use of a free radial artery graft.
Judkins catheters, Amplatz catheters, and other shapes traditionally designed for the femoral approach follow a more “natural” route via the left side of the aortic arch when advanced via the left radial artery. This is considered by some as an advantage over their use via the right radial artery.
Another advantage of distal radial access is the short (two to three hours) haemostasis time, because of the superficial position of this small vessel. If the patient flexes his wrist, haemostasis will not be affected since compression is exerted on the palmar side of the hand. Haemostasis will not result in congestion of the hand since no major veins are obstructed. Haemostasis is relatively mild and very well tolerated by the patients. Short and mild haemostasis and the presence of collaterals to the hand obviate the use of cumbersome patent haemostasis protocols. Finally, this technique can be considered for cases with radial spasm at the wrist level. Ulnar supply may sustain pulsatility in the radial fossa even after spasm is induced by failed radial punctures at wrist level. Having this technique in his/her armamentarium, an operator may theoretically reduce the number of crossovers to other accesses or the need for more proximal radial puncture, which is known to be more risky and difficult due to anatomical issues.
Limitations
The distal radial artery is smaller, making puncture more challenging. A learning curve has to be overcome. In this small series of patients, the distal radial artery was too weak to attempt a puncture at the radial fossa in 23% of cases. However, this also reflects caution on the part of the operator when starting a new technique, for which only the most suitable patients are selected. The number of procedures attempted will increase with improved operator experience. Based on personal communication with A. Babunashvili (Center of Endosurgery, Moscow, Russian Federation), who pioneered distal radial artery access and who performed over 700 procedures (January 2017), ultrasound-guided puncture is recommendable for proper patient selection and a safer and more successful puncture. For sure, this technique is not suitable for unselected patients, for the simple reason that, in a substantial number of patients, no clear pulse is palpable in the anatomical snuffbox. The left distal radial approach will not replace standard transradial access as the default strategy, but it can be considered in patients who prefer or require a procedure via the left arm in the presence of a palpable artery in the anatomical snuffbox.
McNamara et al reported nine patients between 1985 and 1995 with spontaneous thrombosis of the distal radial artery in the radial fossa, all of whom had ischaemic symptoms in the index finger or the thumb11. However, no ischaemic complications using this technique are expected, because the puncture point is located between the ramus palmaris superficialis and the ostium of the princeps pollicis artery. So, there are no conditions for compromising blood flow to the thumb and index finger, also because of the presence of collateral blood supply via the palmar arches. Based on personal communication with A. Kaledin (North-Western State Medical University named after I.I. Mechnikov, St. Petersburg, Russian Federation) who operated on over 2,600 patients (January 2017), and with F. Roghani (Isfahan University of Medical Sciences, Isfahan, Iran), who reported on 108 distal radial procedures (November 2016), no ischaemic events to hand and fingers have been encountered. During an oral presentation at EuroPCR in 2014, A. Kaledin reported an incidence of distal radial artery occlusion of 1.5% in 656 patients treated. Other complications in this series were: haematoma of the wrist and forearm (0.8%), oedema (0.2%), numbness (0.6%), dissection (0.3%), arteriovenous fistula (0.2%), transient ischaemic attack (0.2%), stroke (0.2%), aneurysm (0.2%), and death (0.5%). Of note, no “conventional” radial artery occlusion was noted10.
Although a randomised comparison with the conventional right radial approach would be ideal, even a matched comparison would not be applicable (or possible) in this very small group. Data on the default right radial technique are well known after 25 years. Results of the distal left radial technique in larger series are not yet described but will be in the near future. In addition, the result of this limited experience also includes a definite learning curve, since the artery is smaller, the puncture and wire techniques are different, and other materials are required.
Conclusions
Left distal radial artery access for coronary angiography and angioplasty, in patients with preferred left radial access, is feasible and safe. This new technique deserves serious exploration because it brings advantages to the patients and operators. I consider this new approach to be a further refinement of transradial interventions, which were introduced 25 years ago.
| Impact on daily practice Further refinement and development of the left distal transradial access might offer advantages to the patient undergoing transradial coronary angiography and interventions, while maintaining operator comfort. The risk of radial artery occlusions might be reduced, allowing future transradial procedures if required. |
Acknowledgements
I wish to thank Avtandil Babunashvili (CELT, Moscow, Russian Federation), Alexander Kaledin (North-Western State Medical University named after I.I. Mechnikov, St. Petersburg, Russian Federation) and Farshad Roghani (Isfahan University of Medical Sciences, Isfahan, Iran) for their valuable advice and for sharing their expertise and results.
Conflict of interest statement
The author has no conflicts of interest to declare.

