
Abstract
Aims: The aim of this study was to evaluate whether a real-time (RT) colour pictorial radiation dose monitoring system reduces patient skin and total radiation dose during coronary angiography and intervention.
Methods and results: Patient demographics, procedural variables and radiation parameters were recorded before and after institution of the RT skin dose recording system. Peak skin dose as well as traditionally available measures of procedural radiation dose were compared. A total of 1,077 consecutive patients underwent coronary angiography, of whom 460 also had PCI. Institution of the RT skin dose recording system resulted in a 22% reduction in peak skin dose after accounting for confounding variables. Radiation dose reduction was most pronounced in those having PCI but was also seen over a range of subgroups including those with prior coronary artery bypass surgery, high BMI, and with radial arterial access. This was associated with a significant reduction in the number of patients placed at risk of skin damage. Similar reductions in parameters reflective of total radiation dose were also demonstrated after institution of RT radiation monitoring.
Conclusions: Institution of an RT skin dose recording reduced patient peak skin and total radiation dose during coronary angiography and intervention. Consideration should be given to widespread adoption of this technology.
Abbreviations
BMI: body mass index
CABG: coronary artery bypass grafting
DAP: dose area product
DTS: dose tracking system
FFR: fractional flow reserve
mGy: milligray
PCI: percutaneous coronary intervention
RT: real-time
Introduction
The collective dose of radiation used in medical investigations and procedures increased by more than 700% between 1980 and 20061. Cardiologists are responsible for approximately 40% of the entire cumulative diagnostic radiation dose to the population in the United States2. Interventional procedures are only 12% of all radiological procedures but contribute to approximately 48% of the total collective dose per adult cardiac patient3. Radiation exposure is an important issue for patients but also for the proceduralists, with an interventional cardiologist exposed to two to three times higher radiation per year than a radiologist2,4 .
Exposure to ionising radiation during diagnostic procedures can have dose-related deterministic effects such as skin damage or cataract formation5. There are also random stochastic effects including the risk of malignancy6. It is currently agreed, based upon the “linear no-threshold” model, that no safe dose of radiation exists and that the cancer risk increases linearly with increasing radiation dose2. Therefore, it is generally accepted that every effort should be made to minimise radiation dose to patients and staff7.
During coronary angiography there are a number of well-recognised approaches to reduce patient and operator radiation dose. These include low fluoroscopy frame rates, minimising fluoroscopy time, low image magnification, minimising the distance between the patient and the image detector, collimation and real-time (RT) digital fluoroscopy recording8,9. Also important is minimising operator radiation dose by utilising all available above and below table shielding in conjunction with wearing personal protective equipment such as aprons, lead eyewear and thyroid collars8.
During coronary angiography there is currently no visual cue notifying the operator of a radiation dose that places the patient at risk of deterministic skin effects. Systems which provide RT graphic feedback are designed to prompt alterations in operator behaviour and therefore reduce peak skin dose. The aim of this study was to evaluate whether the use of an RT radiation dose monitoring system reduces patient skin dose and total dose during coronary angiography.
Methods
STUDY POPULATION
All consecutive patients undergoing coronary angiography and percutaneous coronary intervention (PCI) at a single centre (the St. George Private Hospital) from August 2013 to June 2014 were included. Patients were excluded if they underwent structural heart disease interventions, pacemaker implantation or electrophysiology studies. All patients underwent angiography in an Infinix™-I angiography suite (Toshiba Medical Systems, Otawara-shi, Tochigi-ken, Japan).
REAL-TIME SKIN DOSE RECORDING DEVICE
The angiography suite was fitted with a fully integrated dose tracking system (DTS) (Toshiba Medical Systems). The DTS provides an RT pictorial display adjacent to the fluoroscopy image (Figure 1). The display comprises a colour-coded representation of the cumulative skin dose distribution on a patient graphic as well as the RT peak skin dose and cumulative skin dose values at the current RT beam projection. The colour pictorial display changes to yellow when peak skin dose reaches 2,000 mGy and then red when greater than 3,000 mGy.
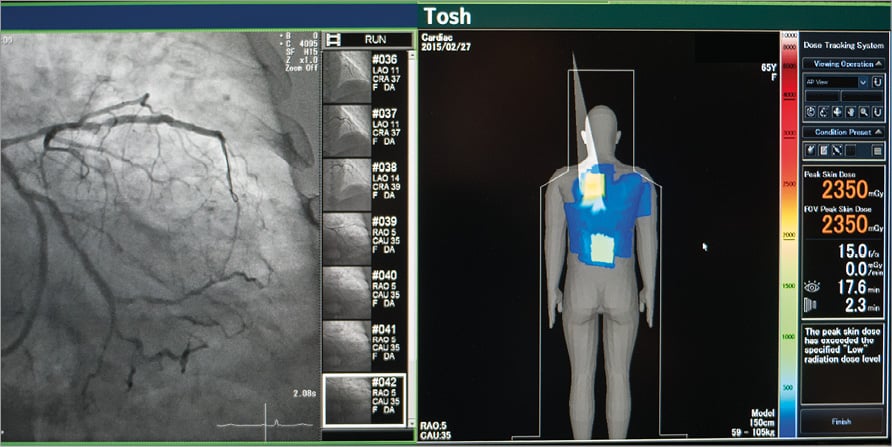
Figure 1. Real-time peak skin dose monitoring system. The dose tracking system (DTS) displays a real-time pictorial and numerical value for cumulative and peak skin dose. The fully integrated system is situated adjacent to the fluoroscopy image and haemodynamic monitoring.
STUDY DESIGN
The study design was a before-and-after non-randomised series of all patients undergoing coronary angiography and percutaneous coronary intervention (PCI). To minimise selection bias from this non-randomised design, the patients represented a consecutive series over a period of nearly one year. As it was a consecutive series of all patients, all patients, including patients who had more than one procedure during this period, were included in the study population. The DTS was recording information on all patients enrolled in the study. Two patient groups were evaluated sequentially for comparison. The control group represented standard clinical practice where the DTS was recording all the procedural variables (including peak skin dose) without the DTS pictorial feedback displayed for the operator: this was referred to as “control”. This represents the “before” feature of the design between August 2013 and December 2013. After the requisite sample size in the “before” group was obtained, a second group, the “DTS group”, was studied with the DTS pictorial feedback displayed for the operator between January 2014 and June 2014.
PROCEDURAL CHARACTERISTICS
Coronary angiography and intervention was performed at the discretion of the operator. Coronary angiography was performed by 16 operators with 10 of them able to perform PCI. No standardised views were recommended and the operator was at liberty to choose all imaging parameters including fluoroscopy frame rate, collimation, filtration, acquisition frame rate, image detector angulation and height, as well as the use of fluoroscopy acquisition.
ENDPOINT DEFINITIONS
Procedural radiation variables were defined as follows:
i) peak skin dose was the highest dose at any portion of the patient’s skin as defined by the DTS; ii) reference point air kerma was the accumulated energy extracted from the X-ray beam per unit mass of air at the predefined interventional reference point (close to the patient’s entrance skin surface)10; iii) cumulative dose area product (DAP) was the dose emitted from the entire X-ray tube; iv) total acquisition duration (acquisition time) was the total duration of cine acquisition used during the case; v) number of cine acquisition exposures was the duration of cine acquisition multiplied by the cine frames per second; vi) fluoroscopy time was the total duration of fluoroscopy used during the case; and vii) contrast volume was the total amount of contrast used.
Significant radiation dose, above which there may be a risk of deterministic complications such as skin injury, was defined as fluoroscopy time >60 mins, reference point air kerma >5,000 mGy and peak skin dose >3,000 mGy10. All operators adhered to standard radiation personal protective equipment.
STATISTICAL ANALYSIS AND SAMPLE SIZE
Sample size calculations were based on observational data from our cardiac catheterisation laboratory in a pilot study of 88 patients. The sample size was calculated on the PCI subgroup, although coronary angiography patient data were collected during the same time period. Assumptions were a mean peak skin dose in the control group of 1,452.5 mGy with standard deviation of 994.1 mGy, an alpha of 0.05, beta of 0.2 and 20% reduction in peak skin dose in the DTS group. Therefore, it was calculated that 187 PCI patients in each group would be required.
Statistical analyses were performed using Stata 14.0 (StataCorp, College Station, TX, USA) and SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Data are expressed as mean±standard deviation (normal distribution) or median±interquartile range (non-normal distribution). Categorical data are presented as frequencies or percentages. For continuous variables correlations were performed using the Spearman and Pearson correlation coefficients. Parametric statistical methods were preferred to non-parametric methods unless assumptions of the former could not be met when the sample size was small11. Graphical representations with scatter plots of variables were constructed to evaluate distributional characteristics, non-linearity and influential outliers.
A repeated measures hierarchical linear mixed effects regression model was considered to be the most appropriate statistical method to analyse the data for three reasons. The first reason was that, because this was a consecutive series of patients, some patients had more than one coronary angiogram and/or PCI, either in the control group or in the DTS group or both. Therefore, a repeated measures analysis was needed. The second reason was that patients were crossed within one or more of 16 operators, as well as crossed by whether the DTS display was turned on or off. Therefore, a hierarchical linear mixed effects regression analysis was needed to adjust for crossing of patient in operator and by DTS off then on display visualisation. The third reason was that the non-randomised nature of the design necessitated adjustment of known confounders of the endpoints of interest, such as peak skin dose. Known confounders included patient age, weight, body mass index (BMI), whether access was radial or femoral, previous coronary artery bypass graft surgery and stent insertion.
Differences between control and DTS groups by patient characteristics, procedural characteristics and operator were compared with repeated measures hierarchical mixed effects logistic regression to model the random effect of operator and because some patients had more than one procedure.
In the repeated measures hierarchical linear mixed effects regression model the primary endpoint, peak skin dose, was the dependent variable in the fixed effect part of the model. The independent variable in the fixed part of the model was group (control or DTS) in the univariate analysis. In the multivariate analysis the fixed part of the model also evaluated patient age, BMI, access, previous bypass grafting and stenting. Operator effects were modelled as random and crossed, using an unstructured covariance matrix. The mixed model estimation used maximum likelihood (ML). Procedural variables were not normally distributed; therefore, they were transformed with a natural log transformation, as this allowed linear mixed model assumptions to be met. The statistical results are back-transformed to facilitate their clinical interpretation.
The primary endpoint was peak skin dose with secondary endpoints of reference being air kerma, acquisition time, fluoroscopy time, number of exposures and contrast use. Differences were considered to be significant at p<0.05.
The study was approved by the local area Human Research Ethics Committee and it also conforms to the guiding principles of the Declaration of Helsinki.
Results
PATIENT POPULATION
From August 2013 to June 2014 a total of 16 operators performed 1,077 consecutive procedures on 1,011 patients. There were 488 procedures in the control group (45%) and 589 procedures in the DTS group (55%). There were 57 patients who had more than one procedure (6%). Six operators performed diagnostic angiography only and the remaining 10 operators also performed PCI, predominantly on an ad hoc basis. There was considerable variability in the number of procedures performed by each operator (from less than 1% to 22%). In only three operators was there a difference in the proportion of patients based upon group. Two operators had a disproportionate number in the control group and one operator more in the DTS group.
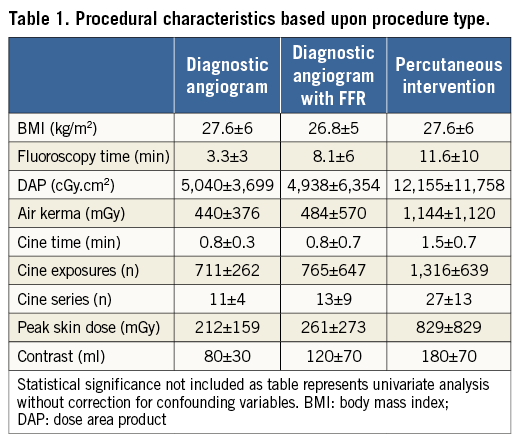
Of the 1,077 procedures, 617 were diagnostic coronary angiography and 460 were coronary angiography and PCI or FFR. A total of 99 (9.2%) procedures (from 97 patients) had prior coronary artery bypass surgery. Overall, 405 (37.6%) procedures were performed via the radial access with the remainder of procedures being performed via the femoral access. Within the PCI group, 47 (10%) had FFR only, 30 (6.5%) balloon angioplasty only, three failed PCI (0.7%) and the remainder (n=380, 83%) stent insertion. When stents were inserted there was a mean of 1.63 per procedure (range 1-6). As expected, PCI procedures had a larger radiation dose compared with diagnostic procedures (Table 1).
Differences between control and DTS groups by patient characteristics, procedural characteristics and operator were compared by repeated measures hierarchical mixed effects logistic regression. There was a significant difference in radial access between the control group and the DTS group (odds ratio 2.1, 95% CI: 1.3-3.5, p=0.004), with more radial access procedures in the DTS group compared to the control. However, this difference was due to four operators performing more radial access procedures over the study time period, reflecting increasing familiarity with radial access over this period. The odds ratio for radial access remained significant when age, BMI, PCI, previous bypass, number of stents and contrast were included in the fixed part of the model (odds ratio 1.55, 95% CI: 1.14-2.12, p=0.005). Demographics and other procedural variables were not different between the control and DTS groups (Table 2) apart from FFR use (control 2.9% vs. DTS 5.6%).
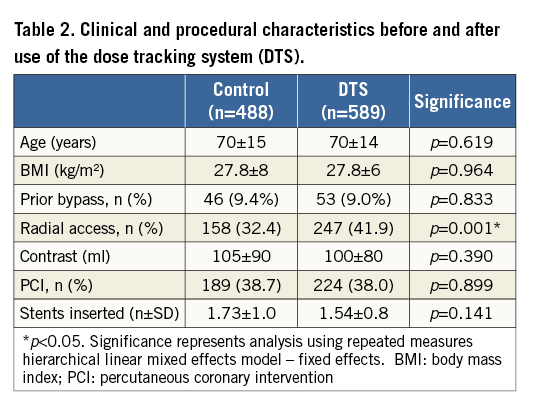
EFFECT OF THE DTS WITHOUT ADJUSTMENT FOR CONFOUNDING VARIABLES
In the entire cohort, institution of the DTS resulted in a significant 15.5% reduction in peak skin dose compared with prior to its use (Figure 2A). There were similar reductions in DAP and reference air kerma with usage of the DTS (Figure 2B, Figure 2C). Compared with control, there was no change in fluoroscopy time (5.0±8.8 vs. 5.9±7.7 min, p=0.507), total acquisition duration (1.0±0.7 vs. 0.9±0.7 min, p=0.636) or number of cine acquisition exposures (864±615 vs. 830±575, p=0.969) in the DTS group.
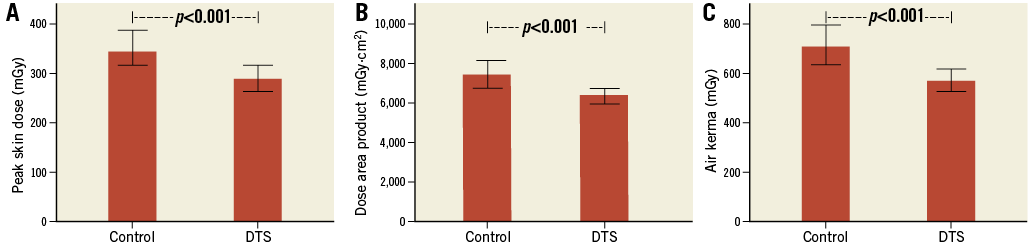
Figure 2. Impact of the dose tracking system. In patients having diagnostic angiography or percutaneous intervention, institution of the DTS resulted in lower peak skin dose (A), dose area product (B) and reference air kerma (C). Bars represent median and error bars 95% confidence interval.
The most profound effect due to implementation of the DTS was radiation dose reduction in those undergoing PCI rather than those having diagnostic angiography. Peak skin dose was reduced by a greater extent in those having stent implantation (46.3%) compared with coronary angiography only (Figure 3). Impressive reductions in other measures of radiation dose were seen in the PCI cohort: fluoroscopy time fell by 14% (12±10 vs. 10.3±8.7 min, p=0.028), DAP fell by 35% (14,531±13,877 vs. 9,501±8,473 cGy.cm2, p<0.001) and reference air kerma by 41% (1,465±1,311 vs. 860±790 mGy, p=0.004).
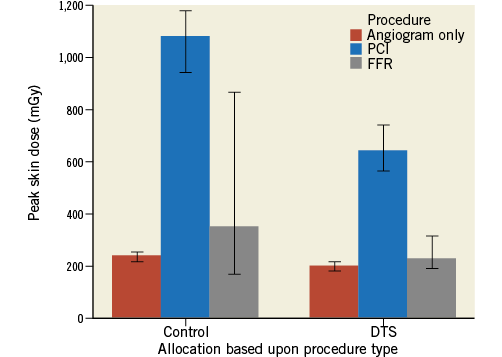
Figure 3. Reduction in peak skin dose due to DTS introduction based upon procedure type. The DTS resulted in an improvement in peak skin dose in patients having both coronary angiography only and PCI. Bars represent median and error bars 95% confidence interval.
Lower peak skin dose was consistent across subgroups including radial access procedures (n=405, 38%, p<0.003), procedures with prior bypass surgery (n=99, 9%, p<0.001) and procedures with patients with high BMI (>25 kg/m2) (n=803, 75%, p<0.001) (Figure 4A-Figure 4C).
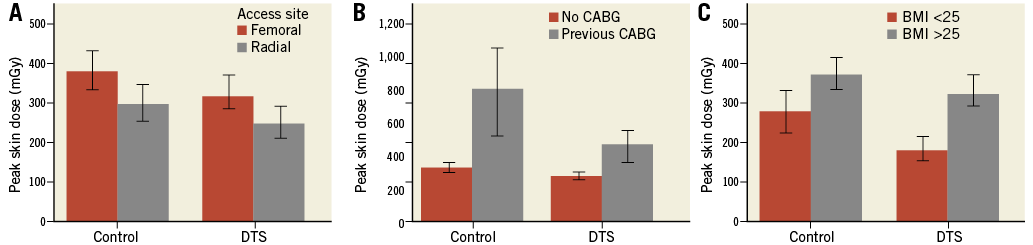
Figure 4. Peak skin dose in patient subgroups. Peak skin dose was reduced by the DTS in patients undergoing the procedure by either femoral or radial access (A), with or without prior CABG (B), and based upon BMI above or below 25 (C). Bars represent median and error bars 95% confidence interval.
In radial access procedures the DTS, compared with control, was also associated with reductions in DAP (5,740±6,013 vs. 6,510±8,597 cGy.cm2, p=0.006), and reference air kerma (497±536 vs. 574±859 mGy, p=0.005).
EFFECT OF THE DTS ADJUSTING FOR CONFOUNDING VARIABLES
In the fixed part of the multiple regression model, PCI and bypass grafting were significant predictors of all procedural radiation variables. BMI was a significant predictor of all procedural radiation variables except total acquisition duration and contrast volume. All other confounding variables were not significant. The DTS remained a significant predictor of peak skin dose, reference air kerma and DAP but not total acquisition duration, number of cine acquisition exposures, fluoroscopy time or contrast volume. Use of the DTS significantly reduced mean peak skin dose by 22% (p<0.001), reference air kerma by 20% (p<0.001), and DAP by 17% (p<0.001) (Table 3). Access site was not a significant predictor of these procedural radiation variables. However, if access site is included in the model as a fixed effect, the use of the DTS significantly reduced mean peak skin dose by 21% (p<0.001). If an access site-DTS interaction is also included, the use of the DTS still significantly reduced mean peak skin dose by 17.5% (p<0.001). Back transformation interpretation of fixed coefficients revealed that peak skin dose was reduced by 22% with the DTS, increased by 5.2% with every one point BMI increase, increased by 384% by PCI and increased by 145% by previous bypass grafting.
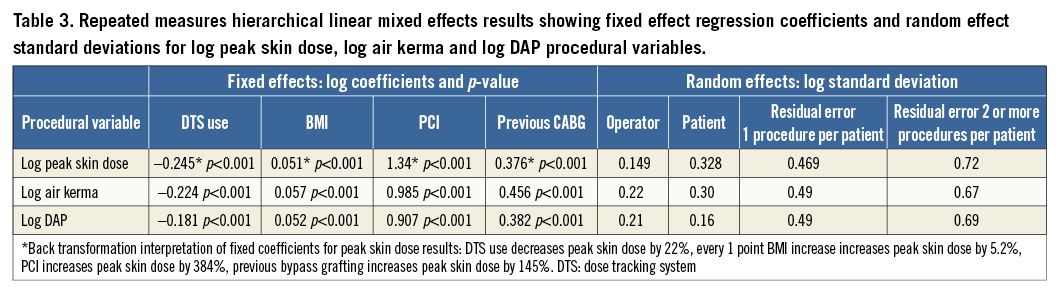
In the random part of the model, operator was modelled as a random intercept. Patients with two or more coronary angiograms and/or PCI were either crossed or nested, or crossed and nested, within operator. Adjusting for all model covariates, operator variability was smaller than between and within patient variability for peak skin dose and reference air kerma. A random coefficient model did not improve fit.
CORRELATION TO STANDARD METHODS
As peak skin dose is a computed cumulative method of radiation dose recording it was correlated against traditionally available measures. There was a good correlation between peak skin dose and the other traditionally available measures of radiation dose (Figure 5).
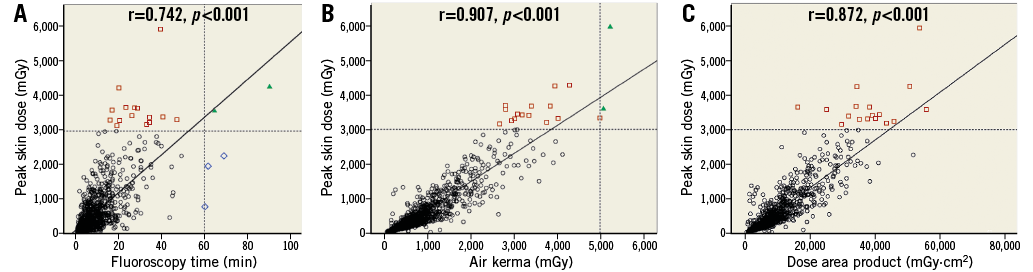
Figure 5. Correlation between peak skin dose and other alternative radiation measures. A) Scatter plot demonstrating moderate correlation between fluoroscopy time and peak skin dose. B) Scatter plot showing good correlation between peak skin dose and reference air kerma. C) Scatter plot showing good correlation between peak skin dose and dose area product. ![]() high risk on peak skin dose;
high risk on peak skin dose; ![]() high risk on fluoroscopy time;
high risk on fluoroscopy time; ![]() high risk on both parameters
high risk on both parameters
HIGH RADIATION RISK PATIENTS
A total of 17 (1.58%) procedures (including one patient on two occasions) were regarded as achieving a high radiation dose during the procedure based upon the peak skin dose definition (>3,000 mGy). A total of five (0.46%) patients were identified as high radiation dose as defined by extended fluoroscopy time and two patients (0.19%) defined by elevated reference air kerma (Figure 5). Fourteen of the seventeen patients regarded as high risk on peak skin dose were not regarded as high risk as defined by fluoroscopy time or air kerma.
Use of the DTS reduced the number of patients identified at high risk of skin damage based upon peak skin dose (control 2.7% vs. DTS 0.7%). Use of the DTS made no difference to the frequency of high radiation dose based upon fluoroscopy time or air kerma. All high radiation dose exposures, irrespective of the definition, occurred in patients undergoing PCI with stent implantation. There were no reports of radiation-related skin effects during routine clinical follow-up.
Discussion
Coronary angiography and intervention are becoming an increasingly important lifetime source of radiation exposure for patients10. Successful performance of these procedures includes attaining technical success with the lowest radiation exposure possible12. Peak skin dose from the DTS is a novel measure to aid in the prediction of dose-related deterministic effects of radiation13. In this study, the DTS resulted in a 22% reduction in peak skin dose during both coronary angiography and intervention after accounting for confounding variables. The benefits of the DTS were seen across a range of patient subgroups, including those with prior CABG, and were particularly evident during stent insertion where there was a 46% reduction in peak skin dose.
The RT feedback provided by the DTS resulted not only in peak skin dose reduction, but also in improvements in measures of overall radiation exposure – DAP and air kerma. This was an unexpected finding and was taken to represent that ongoing RT feedback is a critical factor in successful modification of operator behaviour to reduce radiation dose. This study lacks the ability to confirm the precise alterations to operator behaviour that resulted in these reductions. It is hypothesised that awareness of the peak skin dose prompts working in a different view to avoid an overlapping field of view. When the new position involves less detector angulation, there is likely to be less output from the tube which would therefore reduce DAP and air kerma. Other influences include the greater attention to well-recognised, but often neglected, techniques of radiation minimisation such as assiduous collimation, use of lower frame rates during fluoroscopy and use of fluoroscopy record rather than cine. It has previously been demonstrated that education on radiation protection techniques results in reduced radiation dosing14,15. However, this finding is not universal and, in a recent study of non-RT feedback, a period of education resulted in no difference in radiation dose16.
There was a significant improvement in the number of patients exposed to a peak skin dose which may put them at risk of skin injury. The primary advantage of this technology is that it provides RT pictorial and numeric data that are cumulative during the procedure17. The aim is to alter physician behaviour and improve patient outcome18 by making it possible for reactive dose reduction changes to occur during the procedure. Traditionally, this information is reported as DAP or air kerma, which provides retrospective estimates of the total dose of radiation, rather than localised dose effects.
In a recent study where an audible tracking system was used, a reduced radiation total dose was also observed. However, the authors commented that the increased frequency of beeps during periods of higher radiation dose was distracting to the operator and for this reason it is unlikely that such technology would be adopted more widely19. In comparison, the visual graphic of the DTS is much less distracting and does not detract from the safe performance of coronary intervention.
Peak skin dose demonstrated a moderate correlation with fluoroscopy time and a good correlation with DAP and air kerma in this study. This finding is consistent with the observations of Farah et al who found that maximum skin dose did not correlate with fluoroscopic time but correlated with kerma-area product and cumulative air kerma20. Fluoroscopy time has limited clinical utility as an indicator of radiation dose because it does not reflect patient size, beam angulation, acquisition rate or frame rate21. Reference point air kerma is also limited as it is a cumulative value that represents the dose (close to the skin) if all the radiation were directed at a single location21. It does not account for beam angulation, which is a feature of dose calculation using the DTS.
The limitations in fluoroscopy time and air kerma to determine high risk dose was re-enforced by the fourteen cases in this study with a high peak skin dose that would not have been highlighted on the corresponding modestly increased fluoroscopy time and air kerma.
Based on our findings, it is proposed that the DTS technology may reduce the incidence of deterministic radiation effects and it supports the more widespread use in the setting of invasive cardiac investigation. It has been recommended that patients receiving substantial exposures during cardiac procedures be counselled before discharge and the appropriate arrangements be made for follow-up and monitoring21. Inclusion of peak skin dose and a pictorial display of the exposed area in the procedure report and medical records will alert physicians to the chance of potential complications. Furthermore, such reporting would allow quality control through database analysis – a form of feedback demonstrated to promote safer practices22.
Use of the DTS altered physician behaviour for the reduction of patient skin dose; however, its impact upon scatter and therefore staff radiation exposure was not evaluated in this study. Staff are also at risk of deterministic injury23-25 and possibly stochastic effects26, making occupational exposure an important issue. Altering the image detector angle will result in a reduced peak radiation dose to the patient’s skin, but it is important that the physician is aware of alterations in angulation that could increase scatter to the operator and other staff members. Currently available measures such as the DAP correspond to a greater extent with deterministic and stochastic effects in patients than staff during vascular procedures27,28. Ideally, the institution of the DTS would be accompanied by education on safe dose thresholds, techniques to reduce overall exposure and quantitative scatter plots representing operator exposure. The complete system of radiation safety in the cardiac catheterisation laboratory may include not only the DTS but also RT monitoring of all healthcare workers’ scatter dose.
Limitations
The major limitation of this study was the non-randomised nature of the trial design. The repeated measures hierarchical linear mixed effects regression model adjusted for the known confounding variables of patient age, BMI, arterial access site, previous CABG, stent insertion and the effect of operator. The repeated measures analysis accounted for the few patients who had more than one procedure. However, it is possible that there were unknown confounding variables that would have been better addressed with a randomised trial. One strength of this consecutive trial design was that all patients who met the inclusion criteria and who had procedures in the defined study period were studied. Therefore, the results may be more generalisable to other populations because all operators and their patients were studied. Moreover, if individual patients were randomised, intermittent exposure of the operator to the DTS during the study is likely to cause contamination or a carry-over effect. Behavioural changes in the operators may persist despite randomisation due to intermittent exposure to the DTS. Cluster randomisation by operator is an alternative randomisation method. However, in cluster randomisation a key determinant of patient sample size and success of randomisation is the ratio of operators to patients and, given that this was a single-centre study, there were too few operators.
Conclusion
In conclusion, in this large single-centre study, it has been demonstrated that the DTS is simple to use and results in substantial reductions in important radiation parameters during invasive coronary procedures. A high peak skin dose puts patients at risk of deterministic skin injury, and its routine measurement and reporting are likely to improve patient radiation exposure.
| Impact on daily practice The DTS is simple to use and results in a substantial reduction of patient peak skin and total radiation dose. The greatest gains from DTS are evident during stent insertion, where the patient is exposed to the highest radiation risk. |
Conflict of interest statement
G. Ison received travel support from Toshiba Medical Systems to attend scientific meetings. The other authors have no conflicts of interest to declare.

