- optical coherence tomography
- carotid stenting
Abstract
Aims: Optical coherence tomography (OCT) is an increasingly used intravascular imaging modality to assess coronary stent implantation and vessel healing. A recently developed catheter (C7 Dragonfly™; LightLab Imaging Inc.,Westford, MA, USA) allows visualisation of peripheral arteries up to 10 mm of diameter with high speed pullback. Safety, feasibility and the technique of OCT following carotid artery stenting (CAS) were evaluated in the present study.
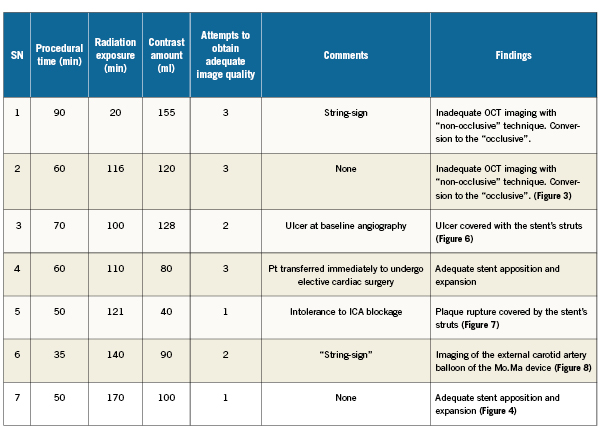
Methods and results: OCT was performed in seven consecutive patients following successful CAS using proximal cerebral protection devices, occluding the common and external carotid artery and gentle manual injection of a total of 8-14 ml of 50% diluted contrast media with normal saline in order to displace the blood from the artery. Once the blood was displaced, pullback was started through the stented segment continuing the gentle contrast injection. After completion of the pullback all injected contrast was aspirated and discarded. In the first three patients, OCT was also performed without internal carotid artery flow blockage. Due to the high blood flow in the ICA it was not possible to replace all residual blood and obtain adequate OCT images. No procedural, in-hospital, or 30-day follow-up complications occurred. One patient experienced transient intolerance to vessel occlusion without any neurological sequels. OCT images were of good quality providing important information regarding stent geometry, presence and lack of strut apposition, plaque fracture, and plaque prolapse.
Conclusions: Intravascular OCT under occlusive proximal protection appears feasible and safe to assess stent implantation in carotid arteries.
Introduction
Optical coherence tomography (OCT) is an increasingly used intravascular imaging modality to assess coronary stent implantation and vessel healing following stent implantation1,2. In vivo imaging with high resolution (approximately 15-20µm) allows visualisation of stent expansion, strut apposition, plaque trauma, and plaque prolapse in great detail3. A recently available OCT catheter (C7 Dragonfly™, LightLab Imaging Inc., Westford, MA, USA) has a maximum scan diameter of 8 to 10 mm, therefore permitting to explore vessels of up to 8mm in diameter, with a faster pullback speed of up to 25mm per second (maximum pullback length 55mm) (Figure 1)4. This catheter may potentially be used for intravascular evaluation of peripheral arteries and peripheral artery stents. In particular, carotid artery stents are of great interest in terms of strut geometry following implantation, plaque coverage, and plaque prolapse. Insufficient plaque coverage and plaque prolapse following stent implantation are not always detectable by angiography, but they have been suggested as the cause of neurologic events during the first 30 days following carotid artery stenting (CAS)5-7. In the following, we report our preliminary experience on OCT following CAS using the new C7 Dragonfly™ LightLab OCT imaging catheter.
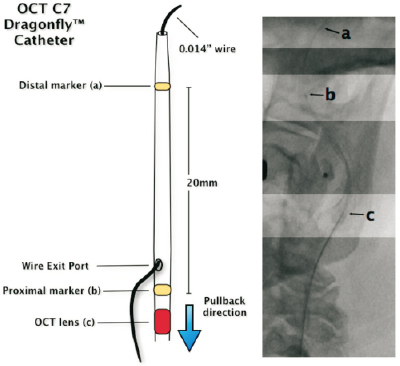
Figure 1. Schematic representation of the C7 Dragonfly™ OCT catheter. At fluoroscopy the operator has to identify three points. a.the marker of the distal tip; b. the proximal marker which indicates the guidewire exit port and the upper distal position of the OCT probe before pull-back; c. the OCT lens which moves from distal to proximal during pull-back (maximum 55 mm at 25 mm/sec).
Patients and methods
Carotid OCT evaluation was performed after CAS procedure had been completed successfully. Both symptomatic and asymptomatic patients were included in the study. The indication for intervention was set by a board certified neurologist according to the degree of internal carotid stenosis and the clinical presentation of the patient, using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (>70% internal carotid artery [ICA] stenosis for the asymptomatic patients and >50% ICA stenosis for the symptomatic ones)8. Severity of ICA stenosis in all patients was calculated with routine preprocedural non-invasive imaging evaluation using multislice computer tomography (MSCT) angiography.
Carotid stenting procedural details
CAS was performed according to standard technique using proximal protection devices9. Proximal embolic protection devices are basically consisting of two high-complaint balloons of different diameters, mounted in the distal part of a guiding sheath placed in the distal part of the common carotid artery (CCA). Both balloons are inflated; one in the proximal part of the external carotid artery (ECA) and the other in the distal part of the CCA, respectively, halting antegrade blood flow towards the brain (“endovascular clamping”). Technical details of the procedures are given in Table1.
OCT techniques in carotids
Optical coherence tomography is a novel intravascular imaging modality that enables high resolution imaging ranging from 10 -20µm. Current OCT systems incorporate advanced near-infrared light emission providing superb tissue imaging resolution. However, red blood cells scatter the OCT infrared light, substantially limiting the quality of the image in presence of blood. Therefore, blood displacement during OCT study, usually with saline or contrast, is necessary to obtain clear images10.
The principal difficulty in performing OCT in the carotid arteries concerns inadequate blood displacement of the blood contained in these large, high-flow arteries in order to obtain satisfactory image quality. In the present study, proximal embolic protection devices with flow blockage (Mo.Ma; Invatec, Rocandelle, Italy; GORE flow reverse system; WL Gore, Flagstaff, USA) were chosen because they offer the unique advantage of complete and reversible blockage of the antegrade flow in the high-blood-flow ICA. That permits complete replacement of stagnant blood with the 50% saline diluted contrast medium (Iodinoxanol 320 Visipaque; GE Healthcare, Cork, Ireland) within the common and internal carotid artery. Blood displacement from the area of interest – in the present case the stented segment of common and internal carotid artery – is considered indispensable for obtaining OCT images of good quality even with the use of the new C7 high-speed pullback OCT catheter. Blood replacement was achieved injecting saline diluted contrast medium either using automatic injection system or by gentle hand syringe injection. The mixture of contrast with saline causes layering inside either the injector container or the syringe. As contrast has a significantly higher molecular weight than saline, using the injector or syringe’s nozzle pointing downwards, the saline is layered at the top of the contrast, thus first allowing the contrast injection followed by saline flush. This technique has been previously used in both invasive and non-invasive MSCT angiography to reduce the amount of contrast given11. OCT computer configuration was performed setting the appropriate parameters according to the contrast and the flush medium utilised. A dedicated technician experienced in handling and operating the OCT device was responsible for the computer set. OCT was performed once the angiographic result of the procedure was regarded satisfactory and any neurological event was excluded. The calibrated C7 Dragonfly™ catheter was advanced just distal to the stent over a 0.014-inch wire which has been used during the CAS procedure.
It was planned to perform OCT imaging with both non-occlusive and occlusive techniques. Non-occlusive (without flow blockage) OCT was performed using the Acist™ CVi automatic injection system (Acist Medical Systems, Eden Prairie, MN, USA). Sixteen millilitres (16ml) of contrast medium (Iodinoxanol 320, Visipaque; GE Healthcare, Cork, Ireland) diluted at 50% with saline were injected at 4ml per second at 600 psi. The pullback was started approximately one second after injection initiation.
For occlusive OCT evaluations both balloons of the proximal protection system were re-inflated after completion of the procedure. Back-flow of blood through the catheter system was carefully monitored and the back-pressure in the ICA was recorded. Subsequently, a 20 ml syringe was used for gentle and continuous manual injection of a total of 8-14 ml of 50% normal saline diluted contrast at approximately 2-3 ml per second (Figure 2). The stagnant blood column present in the distal part of common carotid artery (CCA) and the proximal part of ICA, which represents the region of the implanted stent, was gradually replaced by contrast. Once the persistent contrast staining in the carotid artery was confirmed with fluoroscopy and the real-time OCT images were considered of good quality, pullback was started while continuing the gentle injection. Images were deemed of good quality if they allowed visualisation of the complete vessel area without the presence of blood or artefacts along with easy and accurate identification of vessel wall, plaque morphology and patterns of stent deployment.
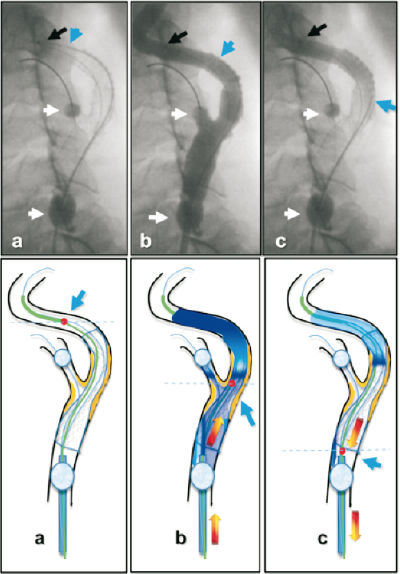
Figure 2. “Step-by-step” OCT evaluation after CAS: angiographic and schematic representation. a. The probe of the OCT catheter is placed well beyond the distal edge of the stent (blue arrow). Both balloons of the proximal protection device, in the common and external carotid artery, are inflated blocking blood flow into the ICA, thus creating a stagnant blood column into the segment containing the stent (white arrows indicate distal (ECA) and the proximal (CCA) balloons inflated). b. Gentle manual injection of approximately 8-14 cc of 50% diluted saline/contrast is performed. During this contrast blood “displacement” the OCT catheter is pulled-back (max. 55 mm pullback in 3 seconds) to obtain images (blue arrow). The red dot indicates the OCT lens during pullback. c.Aspiration of the previously injected saline/contrast.
The OCT team informed the operator when the pullback was finished using both a clearly visible gesture and a vocal notice. As the OCT evaluation was done under complete flow blockage, having both balloons of the proximal protection device continuously inflated, the contrast-saline mixture used to displace blood, remained in stagnant column into the segment of interest. This allows complete aspiration of the contrast-saline mixture through the guiding sheath of the proximal protection device. Once OCT evaluation is completed, all injected contrast was aspirated and discarded through filters in order to exclude the presence of possible debris or thrombi. This process was repeated until good quality images were obtained, up to three times maximum. Close monitoring of the patients was maintained throughout the procedure to detect early possible protection device intolerance or occurrence of neurological events. Device intolerance was defined as an unfavourable change in the level of patient’s awareness (consciousness and motor function) associated with brain hypoperfusion due to flow blockage. Complete device intolerance was defined as a major change in the level of awareness requiring prompt blood flow restoration –with proximal balloon device deflation– and protection device replacement to complete the procedure. Transient device intolerance was defined as minor change in the level of awareness allowing completion of the procedure without protection device replacement. Neurological adverse events were defined as the occurrence of any stroke or transient ischaemic attack (TIA) during the 30-day clinical follow-up period. Cardiovascular adverse events were defined as the occurrence of any myocardial infarction and/or access-site vascular complication during the 30-day clinical follow-up period.
Before deflating the balloons, back-bleeding through the protection system was performed to discard potentially present debris or air. Subsequently, both occlusive balloons in the external and common carotid artery were deflated, ICA antegrade blood flow was restored, and a final angiographic control was performed. Patient monitoring followed routine protocols9.
All patients discharged home and followed-up clinically for 30 days. Double antiplatelet therapy (aspirin 100mg and clopidogrel 75mg, daily) was prescribed in all of the patients for 30 days post procedure; thereafter, aspirin 100mg was recommended indefinitely. Informed signed consent regarding additional intravascular imaging was obtained from all patients.
Results
OCT evaluation has been performed following carotid stent implantation in seven consecutive patients. Clinical and demographic details of the patients are listed in Table1. Four patients were treated with open-cell designed stents, two with closed-cell and one patient was treated with a hybrid stent (Cristallo Ideale, Invatec, Rocandelle [BS], Italy) which combines closed-cell design at the middle portion and open-cell design at both distal portions (Figure3). Procedural time was prolonged for 16±12 minutes. No procedural or in-hospital adverse events were recorded. One patient experienced transient proximal protection device intolerance during OCT and ICA flow blockage (Patient no. 5 in Table1) suffering from progressive loss of consciousness for approximately three minutes. Carotid stenting and OCT evaluation were completed without the need for proximal protection device replacement. The patient regained complete consciousness immediately after ICA flow was restored. No further neurological sequels were noticed in this patient.
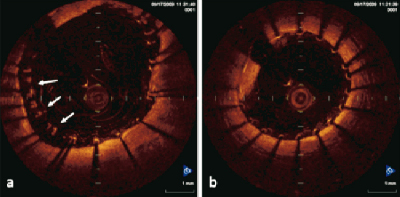
Figure 3. OCT image of a hybrid carotid stent. a. Detailed imaging of the proximal part of the hybrid carotid stent. Non-uniformal expansion of the “open-cell” structure of the stent in the CCA. Note, the non-complete apposition of the struts in the large CCA (arrows) b. circular expansion and apposed “closed-cell” central struts at the site of lesion in the ICA. Formation at 9 o’clock of the figure 3b is probably suggestive of thrombus. CCA: common carotid artery, ICA: internal carotid artery
Imaging evaluation with the C7 Dragonfly™ OCT catheter was achieved in all patients. Images obtained with the occlusive technique and displacement with diluted contrast medium, were of good quality and suitable for further analysis and acquisition. Images acquired with the non-occlusive technique were of inferior quality, not suitable for further interpretation (Figure4). Incomplete blood displacement into the carotid stented segment resulted in significant scattering of the OCT infrared light, producing large amounts of artefacts and creating false images unable to be interpreted correctly or allowing for detailed measurements. OCT catheter advancement and manipulation was easy in all cases.
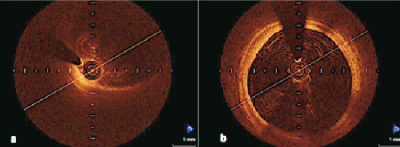
Figure 4. Appearance of residual blood during OCT imaging after carotid stenting using the non-occlusive technique (a). Inadequate quality for interpretation. Note the difference in quality of images in the same patient between non-occlusive and occlusive technique (b).
Information regarding the acute result after carotid stenting, the stent geometry, and plaque coverage could be obtained in all patients. In two patients, a small plaque prolapse was identified after stent implantation which was not angiographically visible (Patients numbers 3 and 6 in Table1, Figure 5). Both patients had undergone uncomplicated CAS in soft atheromatic plaques –as had been determined in the routine preprocedural duplex ultrasound– and remained asymptomatic post procedure.
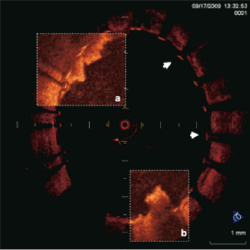
Figure 5. OCT image after CAS with a closed-cell designed stent. Note homogenous circular distribution of the stent’s struts. Frame a and b have been interpreted as plaque prolapse between two stent struts.
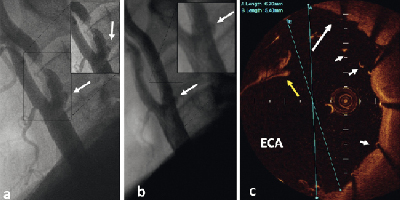
Figure 6. a. Baseline angiography of the patient #3 b. Final angiographic result of the patient following CAS with open-cell designed stent. The arrow indicates a small ulcer at site of CCA/ICA bifurcation. c. OCT image of the site of interest shown in angiography. The white arrow indicates the ulcer covered by the stent’s struts. The yellow arrow indicates artefact from remaining blood. The arrowheads indicate stent’s struts. The maximum inner diameter of the vessel at this point is 5.4 mm. Blue lines A and B represent measurements of the vessel diameter (correct B).
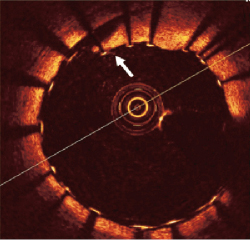
Figure 7. OCT image of a symptomatic patient following carotid stenting and dilatation with a balloon. Localised vessel dissection caused by the stent implantation and post-dilatation adequately covered by the stent struts (open-cell design) (arrow).
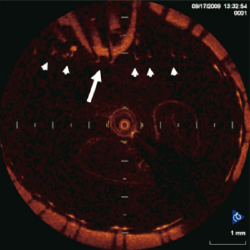
Figure 8. OCT image of the common carotid artery with the occlusion-balloon of the ECA still behind the struts of the stent (arrowheads). Following removal of the balloon the self-expandable stent theoretically will appose to the vessel wall.
Clinical follow-up at 30 days post-procedure was obtained in all patients. None of the patient experienced neurological or cardiovascular adverse events.
Discussion
In this present study, we performed OCT imaging in a small series of patients after carotid stenting using two different techniques (occlusive and non-occlusive). All patients underwent successful carotid stenting using proximal protection devices without major complications, while OCT provided unique images of the stented carotid arteries. Moreover, proximal embolic protection devices were used to achieve blood flow blockage, and subsequently blood displacement, into the stented segment of the carotid arteries, something which proved to be mandatory in order to obtain OCT images of good quality. Clinical application of this imaging technique in human carotid arteries has been previously performed just once, to detect the presence of intraluminal thrombus inside ICA in a patient prepared for carotid stenting, but eventually underwent carotid endarterectomy12. To our knowledge, this is the first report on the clinical experience of OCT evaluation of the stented segment of ICA, in a series of patients who underwent carotid stenting. Additionally, it describes and proposes a suitable technique to obtain good quality OCT images in carotid arteries.
The newly developed OCT catheter (C7 Dragonfly™; LightLab Imaging Inc., Westford, MA, USA) allows the visualisation of larger arteries –up to 8mm in diameter– retaining an adequate vessel-wall penetration. This OCT catheter offers the great opportunity of visualisation of stented carotid arteries in order to gather important information regarding stent strut apposition, plaque coverage and plaque prolapse. Inappropriate stent apposition and plaque prolapse may not be seen during angiography in carotid stenting. OCT evaluation after carotid stenting may help identify suboptimal results not easily identified with angiography. This could help to better understand the potential role of previously unrecognised variables, like small plaque prolapse, stent malapposition and patterns of plaque coverage, in the short- and long- term outcome of the patients undergoing carotid stenting. All these factors have been the subject of speculations in order to correlate with future adverse events after carotid stenting but have never been proven as they have not, until now, been studied in detail5-7.
To obtain good quality OCT images from coronary arteries, blood displacement with saline is mandatory. Therefore, the methodology widely used consisted in balloon occlusion and saline injection into the coronary artery to displace the blood with saline (an occlusive technique). Recently, a non-occlusive technique was introduced by Prati et al according to which continuous manual injection of contrast medium could effectively replace intracoronary blood and provide OCT images of good quality, comparable to those using the occlusive technique13,14. The fast pullback of the new C7 Dragonfly™ OCT catheter favours these non-occlusive techniques. In the present study we initially tried to perform post-carotid stenting OCT using the non-occlusive technique without success. It appears that, unlike the coronaries, in the high-flow and large diameter carotid artery, it is almost impossible to replace all of the blood. Thus, the OCT images obtained were inappropriate for further study and acquisition. This technique was abandoned after the first three patients. Efforts to improve image quality in non-occlusive techniques, such as the application of higher injection flow-rates or the use of undiluted contrast medium, proved unsuccessful, they also carry the potential risk of complications due to excessive contrast administration. Proximal protection devices offer the great advantage of an occlusive system, allowing blood flow blockage and subsequently blood replacement with contrast medium. With this technique, we obtained images of high quality suitable for detailed study and acquisition. Moreover, the high pullback speed of the OCT C7 catheter allows for shorter occlusion times making the OCT process less time-consuming, while reducing the possibility of protection device intolerance. If ICA flow blockage is well tolerated, OCT can be repeated in order to achieve the best quality images. Another advantage of the occlusive system is that the contrast medium injected can be aspirated through the proximal protection device and discarded, thus minimising the risk of contrast induced nephropathy and encephalopathy.
As shown by the present study, performing OCT after carotid stenting using proximal protection devices appears safe. To maintain high levels of safety some important technical details must be followed. It is essential that before starting the OCT process carotid artery stenting must have been completely finished, leaving the proximal protection device in place. Before reactivating the protection device by inflating the balloons in the external and common carotid arteries, all possible dislodged debris must have been previously aspirated and discarded. Fluoroscopic observation during careful injection of contrast medium is critical. We recommend injecting at the minimally necessary pressure the minimally necessary amount of diluted contrast to replace the column of blood with contrast. Notably, high levels of cooperation between the operators and the OCT team is crucial in order to achieve success in these complex procedures.
Limitations
The relatively small number of patients is an important limitation of this study. Additional patients are needed to confirm the clinical efficacy and safety of this novel imaging technique of stented carotid arteries. Moreover, due to the small number of patients, we were not able to identify any clinical or anatomical variable in which the non-occlusive technique would be preferable to the occlusive one. However, it is possible that particular clinical variables and specific anatomical configurations may favour one technique over the other.
OCT evaluation of the carotid plaque before stenting was not performed during this initial experience. Therefore, comparison of the images acquired after stenting to those pre-stenting, was not possible.
OCT after carotid stenting regard a relatively challenging procedure requiring additional procedural steps and probably repeat flow blockage attempts. Therefore, it should be performed in experienced centres capable of prompt recognition and management of complications. Excellent familiarity with the usage of proximal protection devices and OCT catheter manipulation should be considered mandatory.
Conclusions
OCT after carotid stenting appears feasible and safe without the need of additional contrast medium. Our preliminary data suggest that using the occlusive technique, better quality images were obtained with the advantage not to increase the contrast load. Non-occlusive techniques may need further refinements. Carotid OCT allows collection of important data regarding the stent and plaque behaviour providing information on potentially significant details not seen with standard angiography. The clinical value of this information and its impact on future stent designs has to be clarified in further studies with larger number of patients.
Conflict of interest statement
The authors have no conflicts of interests to declare.
1.Tanigawa J, Barlis P, Di Mario C. Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention 2007;3:128-36.
2.Prati F, Zimarino M, Stabile E, Pizzicanella G, Fouad T, RabozziR, Filippini A, Pizzicanella J, Cera M, De Caterina R. Does optical coherence tomography identify arterial healing after stenting? An in vivo comparison with histology, in a rabbit carotid model. Heart 2008;94:217-21.
3.Agostoni P, Stella PR. Optical coherence tomography: new (near-infrared) light on stent implantation? Heart 2009;95:1895-6.
4.Bezerra H Tahara S, Parikh S, Costa M. “Old” problems revisited; imaging the coronary in high resolution. EuroIntervention 2009;5:523-5.
5.Takigawa T, Matsumaru Y, Kubo T, Fukuhara N, Hayakawa M, Usui M. Recurrent subacute in-stent restenosis after carotid artery stenting due to plaque protrusion. Neurol Med Chir (Tokyo) 2009;49:413-7.
6.Aikawa H, Kodama T, Nii K, Tsutsumi M, Onizuka M, Iko M, Matsubara S, Etou H, Sakamoto K, Kazekawa K. Intraprocedural plaque protrusion resulting in cerebral embolism during carotid angioplasty with stenting. Radiat Med 2008;26:318-23.
7.Chakhtoura EY, Hobson RW 2nd, Goldstein J, Simonian GT, Lal BK, Haser PB, Silva MB Jr, Padberg FT Jr, Pappas PJ, Jamil Z. In-stent restenosis after carotid angioplasty-stenting: incidence and management. J Vasc Surg 2001;33:220-5.
8.NASCET Investigators. North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711-720.
9.Reimers B, Sievert H, Schuler GC, Tübler T, Diederich K, Schmidt A, Rubino P, Mudra H, Dudek D, Coppi G, Schofer J, Cremonesi A, Haufe M, Resta M, Klauss V, Benassi A, Di Mario C, Favero L, Scheinert D, Salemme L, Biamino G. Proximal endovascular flow blockage for cerebral protection during carotid artery stenting: results from a prospective multicenter registry. J Endovasc Ther 2005;12:156-65.
10.Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, AlbertucciM. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 2007;3:365-70.
11.Cao L, Du X, Li P, Liu Y, Li K. Multiphase contrast-saline mixture injection with dual-flow 64-row MDCT coronary CTA. Eur J Radiol 2009;69:496-9.
12.Yoshimura S, Kawasaki M, Hattori A, Nishigaki K, Minatoguchi S, Iwama T. Demonstration of Intraluminal Thrombus in the Carotid Artery by Optical Coherence Tomography: Technical Case Report. Neurosurgery 2010;67;onsE305
13.Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Giudice M, Propris SD, Albertucci M. From bench to bedside: a novel technique of acquiring OCT images. Circ J 2008;72:839-43.
14.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW; Expert’s OCT Review Document. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401-15.

