
Abstract
The left main coronary artery (LMCA) is responsible for supplying the majority of the left ventricular myocardium. Visual estimation of stenosis severity on angiography has major limitations and methods to assess functional significance, such as fractional flow reserve (FFR), have been shown to yield better outcomes; however, to date, major trials examining the use of such physiological indices have excluded LMCA disease. Furthermore, LMCA disease commonly co-exists with downstream disease, which complicates the interpretation of coronary physiological data. This review summarises existing evidence for physiology-guided management of LMCA disease. It will also explore the difficulties posed when functionally assessing LMCA lesions and outline potential solutions. Finally, we aim to provide insight into how novel physiological tools may improve the management of LMCA disease in the future.
Introduction
Left main coronary artery (LMCA) disease is discovered in 5-8% of patients undergoing coronary angiography1. It has traditionally been associated with poor prognosis as it can result in reduced blood flow to a large portion of the myocardium1.
It is now well known that the angiographic estimation of the severity of a stenosis is a poor determinant of its functional significance2. Two-dimensional (2D) angiography of the LMCA is hampered particularly due to lesion angulation, lack of reference vessel, eccentricity, bifurcation anatomy, overlapping branches and foreshortening3. As a result, the misclassification of LMCA lesion severity by angiography alone is even higher than for other vessels, with misclassification rates approaching 50%2,4. Despite this, almost the entire evidence base of LMCA management is based on visual angiographic estimation alone. The recently concluded LMCA revascularisation trials, EXCEL and NOBLE, exemplify this, with decisions to revascularise based on angiographic appearance alone5,6.
The importance of ischaemia assessment has become increasingly evident over the last two decades7, with fractional flow reserve (FFR), the ratio of distal coronary (Pd) to aortic pressure (Pa) during maximal hyperaemia, emerging as a standard of care for assessing coronary stenoses in the catheterisation laboratory, providing a surrogate for ischaemia testing at the point of diagnostic angiography. Although several large randomised trials have demonstrated the utility of FFR in guiding revascularisation8-10, these trials have excluded patients with LMCA disease. Consequently, the growing trend for physiology-guided management of more distal lesions is not matched for LMCA disease, with the bulk of LMCA research continuing to focus on identifying the modality of revascularisation rather than whether we should intervene in the first place5,6.
Beyond angiographic assessment of the LMCA: fractional flow reserve
The seminal validation study of FFR for the identification of myocardial ischaemia was performed in stable patients against a combination of non-invasive ischaemia tests, but only enrolled two patients with LMCA disease11. Since the inception of FFR, there has been mounting evidence suggesting that physicians are poor at identifying physiologically significant lesions by angiography alone2,4. A recent study of over 4,000 coronary lesions with both angiography and FFR found that coronary angiography alone misestimates physiological severity when using an FFR threshold of 0.8 in over one third of angiographically intermediate stenoses2. This observational study included 152 patients with LMCA disease and found visual-functional mismatch to be just as common, with a particularly high lesion underestimation. These data are accompanied by growing evidence from trials demonstrating improved outcomes by FFR-guided PCI versus angiography alone– these have all excluded LMCA disease8,10,12.
Hamilos et al reported the largest observational study on the utility of FFR in LMCA lesion assessment13. Patients with angiographically equivocal LMCA disease who underwent adjunctive pressure wire assessment were enrolled. Patients with FFR >0.80 were managed with medical therapy and those with an FFR ≤0.80 underwent CABG. They found equivalent clinical outcomes in both groups, suggesting that an LMCA FFR threshold of 0.80 may be used to defer revascularisation. A recent meta-analysis of observational data (525 patients from six studies) drew similar conclusions (Figure 1)14.
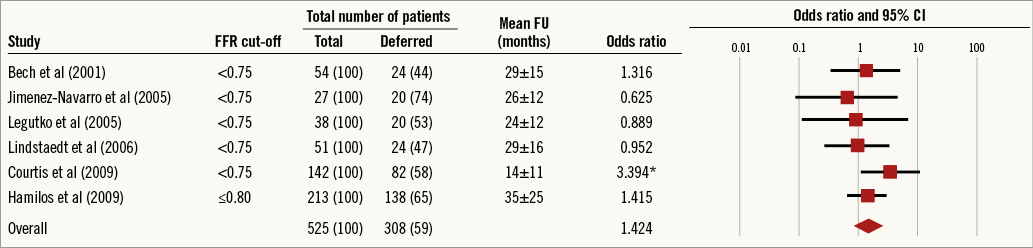
Figure 1. Summary of results from meta-analysis of combined long-term outcomes in FFR-guided management of LMCA disease. *denotes p<0.05.
Several criticisms can be made of the current data supporting FFR-guided management of LMCA disease. The studies did not use a uniform FFR threshold, hampering extrapolation to contemporary practice. In addition, observational data are prone to the pitfalls of non-randomised data, including selection bias, which cannot be overcome by meta-analyses. An argument against the use of FFR to guide LMCA revascularisation is that the risk of plaque rupture events may not be related to the haemodynamic significance of a stenosis but is instead determined by underlying plaque composition. In other words, deferring revascularisation of the LMCA on the basis of an FFR >0.80 may leave a group of patients at risk of myocardial infarction. Although there has been no signal of excess MI/death in the vast majority of FFR trials, the recently presented FUTURE trial (NCT01881555), which is discussed in detail later in this review, did include LMCA disease in 11% of patients, with a signal for excess mortality in the FFR-guided arm. Whilst we await robust randomised data on FFR in LMCA disease, the FUTURE trial data lend support to the idea that the potential consequences of LMCA plaque rupture are far greater than a more distal plaque rupture event, and the observational FFR data of LMCA revascularisation to date are probably underpowered to detect the impact of such events.
Theoretical challenges to physiological assessment of the LMCA
Coronary blood flow is related to the size of the viable myocardial bed subtended15. FFR takes account of the myocardial mass perfused as it is the ratio of blood flow to a given mass of tissue in the presence of a stenosis (Qs) to blood flow to the same mass of tissue if the vessel were unobstructed (QN): whereby FFRmyo=Qs/QN. The LMCA territory constitutes the vascular beds of the left anterior descending (LAD) and left circumflex (LCx) arteries16 and, in the absence of obstructive disease in each branch, the ratio of flow in each is determined by the resistances of their respective vascular beds. As the minimal resistance in a vascular bed can be assumed to be independent of any upstream stenosis17, the FFR of an LMCA stenosis should be identical, whether measured in the LCx or LAD. However, in the presence of any downstream disease, FFR will be determined by the LMCA stenosis as well as disease in each limb. Given that atherosclerosis tends to affect the whole coronary tree, LMCA disease is usually accompanied by additional downstream disease in the LAD and/or LCx1. In such cases, the LMCA and distal stenosis should be considered to act as serial stenosis18,19.
Ischaemia assessment of individual lesions in the presence of serial stenoses is problematic because of the complex interdependence of each lesion resulting from altered fluid dynamics within the vessel that affects their relative severity. This haemodynamic interplay is most challenging when measuring LMCA FFR with increasingly severe distal disease. This is because we know from seminal work by Gould et al that coronary blood flow is reduced, even with intermediate stenosis, during hyperaemic conditions20, and that this flow reduction is accompanied by increasing laminar flow separation within the vessel with increasingly severe reductions in luminal diameter (Figure 2). The presence of disease distal from the LMCA is generally associated with underestimation of stenosis severity, and may lead to inadvertent deferral of LMCA revascularisation21. Considering the potential influence of distal disease on clinical decision making in the critical setting of LMCA stenosis, care should be taken to identify carefully the presence and severity of coronary artery disease distally before drawing conclusions on the functional significance of LMCA stenoses.
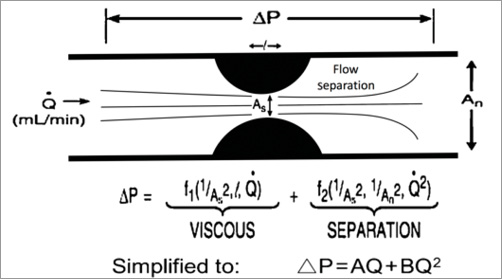
Figure 2. The pressure gradient induced by stenosis can be described by a quadratic relationship, combining the laws of both Poiseuille and Bernoulli, to describe the concomitant energy losses by viscous friction (f) and flow separation (s).
The problem of serial stenosis interplay also exists with a disease-free side branch (e.g., LMCA stenosis co-existing with a stenosis in the mid LAD but with a disease-free LCx). Whilst potential interaction is recognised, the practice of measuring LMCA FFR by placing the wire in the disease-free daughter vessel has been shown to be a reliable method in in vitro studies with only minimal interference from downstream disease in the diseased branch19,22,23. Fearon et al went on to demonstrate this in their in vivo study by interrogating LMCA stenoses in 25 patients following PCI of the LAD±LCx. FFR was measured in the LAD and LCx before and after creation of downstream lesions by inflating balloons within newly placed stents. They then compared “true” FFR measured in the disease-free vessel with the “apparent” FFR measured in the diseased vessel. They found that the true LMCA FFR was significantly lower than the apparent FFR (0.81 vs. 0.83, p<0.001). Although the difference existed in this continuous variable, the authors interpreted this as clinically insignificant and at the upper end of the natural test-retest variability of FFR. They concluded that, in most cases when there is a disease-free branch of the LMCA, downstream disease within the other branch does not have a clinically significant impact on LMCA FFR with the pressure wire positioned in the disease-free vessel, unless disease within this branch is very severe (FFR <0.45), and that an FFR value of >0.85 in the disease-free side branch would mean that the LMCA lesion can be safely assumed to be functionally non-significant19. Whilst this seems reassuring, there remains concern that any significant difference in the continuous variable of FFR may be associated with important outcome differences. Whilst these studies are small and prone to theoretical flaws, for the purpose of LMCA disease with serial disease in only one downstream daughter vessel this method is, for now, an acceptable solution for this unique case of serial stenosis interplay.
Best practice in LMS physiological recordings
GENERAL TECHNICAL CONSIDERATIONS
Physiological assessment in general mandates meticulous preparation with zeroing of aortic pressure, equalising the guide catheter pressure with wire pressures before seating the guide catheter in the LMCA. However, since the guiding catheter could obstruct flow, particularly for ostial LMCA lesions, it should be disengaged once hyperaemia is induced, to reduce the effects of “pressure damping”. If this is proving difficult, then the use of an additional wire within the other branch can be used to stabilise the distance from the LMCA ostium.
For induction of hyperaemia, a continuous intravenous infusion is recommended because, when unseating the guide catheter, adequate administration of adenosine to distal microvasculature can be otherwise challenging. In addition, the use of intravenous adenosine also enables an FFR pullback manoeuvre. Intravenous adenosine infusions, particularly when entering from the peripheral venous circulation, can produce several patterns of Pd/Pa response24. In order to overcome such variability, Johnson et al identified a stable period during adenosine-induced hyperaemia, called the “smart minimum’’ (lowest “5 cardiac cycle” average), that was found to be most reproducible24. Furthermore, it is important to remember that patients with LMCA disease often have contractile impairment which can result in elevated central venous and right atrial pressures. Where available in such patients, it should be remembered that FFR assumes venous pressure is negligible and, when measured venous pressures are significantly elevated, values need to be interpreted cautiously25.
“ISOLATED” LEFT MAIN STENOSIS
In the scenario of isolated LMCA disease (or “LMCA equivalent” with contiguous downstream disease), there is no theoretical difference in measuring LMCA FFR when the wire is placed in either branch. There is still sufficient reason, however, to perform an FFR pullback manoeuvre from both the distal LAD and LCx (Figure 3) because the significance of downstream disease should not be presumed from visual estimation alone2,26.
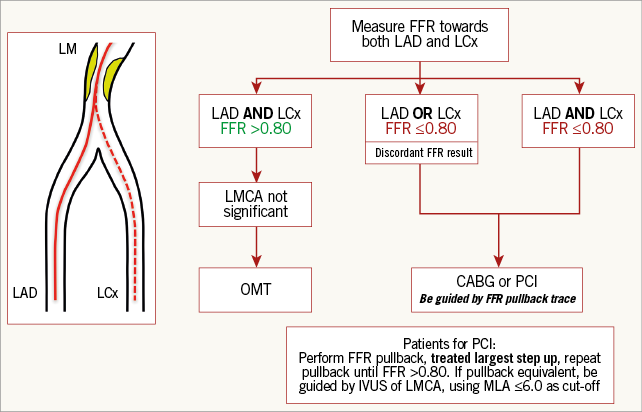
Figure 3. A suggested decision-making algorithm when confronted with LMCA disease, taking into account various combinations of downstream disease.
LEFT MAIN STENOSIS AND EPICARDIAL DISEASE IN ONE DOWNSTREAM BRANCH
When, concomitant to an LMCA stenosis, a significant stenosis is present in only one downstream branch, measuring the true FFR is still potentially confounded. As described in the theoretical challenges section, whilst not fully validated, the practice of measuring LMCA FFR by using a disease-free daughter vessel has been shown to be relatively reliable with only minimal interference from downstream disease in the diseased branch unless severe (>90% diameter stenosis or FFR <0.45)19,22,23, in which case an LMCA FFR of 0.85 can be taken as reassuring. Whilst these studies generally support the use of a disease-free side branch in functionally assessing LMCA severity, the clinical relevance of applying an LMCA FFR grey zone in daily clinical practice is limited, with a contemporary 0.80 threshold still recommended when assessing LMCA FFR using a disease-free side branch (Figure 3).
LEFT MAIN STENOSIS AND DISEASE IN ALL DOWNSTREAM EPICARDIAL BRANCHES
In the absence of a disease-free branch, serial lesion interplay becomes particularly challenging with a significant likelihood that distal stenoses can alter flow and, in turn, the pressure gradient across the LMCA18. At present, in patients having LMCA PCI, the practice of treating the greatest FFR following manual FFR pullback appears to be the best available method and has been shown to be safe and to reduce unnecessary revascularisation with acceptable outcomes at a mean follow-up period of nine months21. We therefore recommend that, for any LMCA stenosis, the pressure wire should be advanced distally in both epicardial branches to determine both FFR values. Following this, a fixed rate pressure wire pullback should be performed in both branches to allow estimation of the physiological significance and length of all lesions. Recommendations following these pullback manoeuvres are summarised in Figure 3. Information from this physiological assessment should lead to all revascularisation strategies being considered, not just PCI, particularly in the context of diabetes or high SYNTAX score and with the help of a Heart Team discussion.
Applicability of alternative physiological measures in LMCA
Several alternative physiological measures of functional stenosis severity have been introduced and are gaining ground in clinical practice. These include indices derived during resting conditions, namely instantaneous wave free ratio (iFR) and resting Pd/Pa.
iFR is a pressure-derived, adenosine-free index that assesses the pressure gradient during the terminal 75% of diastole, when microvascular resistance is purportedly minimal. Validation of iFR has been based on demonstration of reasonable agreement with FFR across several studies27-29. It has recently been shown to have comparable outcomes to FFR in two non-inferiority trials that compared iFR-guided revascularisation versus FFR-guided revascularisation30,31. Whilst the index continues to be a source of debate, iFR has been purported to be affected less by serial lesion interplay compared to FFR, as it is measured without hyperaemia when haemodynamic interplay may be more significant32. Given the frequent occurrence of downstream serial disease, iFR may have potential application when assessing LMCA stenoses, although supporting data are currently lacking. While we continue to await the outcome of randomised trials comparing iFR-guided versus FFR-guided PCI, it is worth noting that these trials continue to exclude LMCA disease. If further data emerge to suggest non-hyperaemic pressure-based indices have particular value in assessing serial lesions, resting Pd/Pa may also have potential utility for LMCA assessment, especially with co-existent downstream disease. Once again, there is little current evidence to support this assertion, and the value of these alternative indices remains largely unsettled within this unique subset of patients.
Recent and upcoming trials examining physiology-guided LMCA management
While there are several ongoing trials of FFR and iFR, it is notable that virtually all of these exclude LMCA disease. Furthermore, trials comparing surgical versus percutaneous revascularisation have continued to rely on angiographic rather than physiological diagnosis of LMCA disease5,6. In the FAME 3 trial (NCT02100722), patients with multivessel disease are randomised to CABG or FFR-guided PCI and patients with significant LMCA disease are excluded. The same applies to the ongoing SYNTAX II trial (NCT02015832) that also evaluates CABG versus physiology-guided PCI in patients with multivessel disease using a hybrid iFR-FFR approach. The DEFINE-FLAIR trial (NCT02053038), comparing clinical outcomes of iFR-guided PCI versus FFR-guided PCI, also excludes LMCA disease. The iFR-SWEDEHEART trial (NCT02166736), with a similar design to DEFINE-FLAIR, does not specifically exclude LMCA, leaving some opportunity for future substudies.
The FUTURE trial (NCT01881555) recently reported results and did not exclude LMCA. This trial, of acute and stable patients with multivessel disease, included 11% with LMCA involvement. This trial of 836 patients was stopped early, before its enrolment target of 1,700 patients, as the risk of all-cause mortality was more than double in the FFR-guided PCI arm (p<0.02). Whilst we await the full manuscript, there is ongoing debate regarding why there was this increased mortality signal in the FFR-guided PCI arm. Of interest, the excess mortality did not continue once the trial was stopped at 12-month follow-up, suggesting that this result may have been “noise”. In addition, some baseline characteristics varied (e.g., diabetes and SYNTAX score). Whilst some of these criticisms seem fair, the fact that a significantly greater proportion of LMCA patients was included than in previous physiology-guided PCI trials certainly invites questions regarding the benefit of FFR-guided revascularisation in higher-risk groups, and adds further weight to the demand for a randomised trial examining physiology-guided LMCA revascularisation.
Imaging as a surrogate of coronary physiology
INTRAVASCULAR ULTRASOUND (IVUS)
IVUS-guided PCI has been associated with better outcomes, due to improved vessel sizing for optimal stent deployment33. It is therefore attractive also to use intravascular imaging as a diagnostic tool if sufficient correlation to physiological measures can be found. As such, several studies have assessed the use of minimum luminal area (MLA), derived from IVUS values, for inferring the functional significance of LMCA disease. Jasti et al were the first to show this correlation in a study of 55 patients34. Perhaps due to the smaller perceived variation in LMCA length, diameter and eccentricity, there is better concordance between IVUS and FFR in the LMCA compared to other vessels35. Subsequently, other studies have demonstrated the relationship between the degree of MLA reduction and subsequent MACE rates, with the prospective multicentre LITRO study showing that an IVUS MLA of 6 mm2 could be used for safely deferring LMCA revascularisation, yielding acceptable long-term clinical results36. Despite such data, there remains significant variability regarding which IVUS cut-off values correlate best with FFR.
Apart from the impact of demographics on the normal reference range, this significant variation in IVUS MLA values can be explained physiologically, because the pressure drop across a narrowing is governed by several other factors in addition to the MLA (Figure 2). Nonetheless, the literature does suggest that using IVUS thresholds in the region of 4.5-7.5 mm2 provides some improvement to angiography alone in determining the functional significance of an LMCA stenosis, provided a true LMCA cross-sectional cut is obtained37,38. It is important to keep in mind that the studies supporting using thresholds as low as 4.5 mm2 were conducted in populations with only Asian patients, who may have relatively small hearts, with the authors of the study recognising that the global applicability of the results may be limited37.
OPTICAL COHERENCE TOMOGRAPHY (OCT)
OCT offers greater spatial resolution at the cost of tissue penetration, and thus potentially clearer delineation of the intima-luminal limit, with data showing more accurate and reproducible assessment of lesion diameter when compared to IVUS using phantom stenosis models39. Whilst there are no data correlating OCT-derived LMCA luminal dimensions with FFR, there is a suggestion from a study by Gonzalo et al40 that OCT is moderately efficacious versus IVUS in identifying haemodynamically severe coronary stenoses in smaller epicardial vessels with an MLA <3 mm2. However, as with other studies attempting to use intravascular imaging as a surrogate for physiology, they found specificity levels were too low to use it as a substitute for FFR for functional assessment.
Conclusions
Considerable randomised data exist supporting physiology-guided revascularisation in epicardial vessels, but the evidence base largely excludes LMCA disease. The complex anatomy and physiology of LMCA lesions makes FFR assessment more challenging, particularly with regard to downstream serial stenosis interplay. We have suggested a simple algorithm involving FFR pullback manoeuvres from both the LAD and LCx branches, with aid from intravascular imaging if further clarification is needed in equivocal cases, that will provide diagnostic clarity in most scenarios. Whilst non-randomised data suggest that physiology-guided management is valid and equally effective for the LMCA, we await randomised controlled trial data to support its widespread use.
| Impact on daily practice The considerable randomised evidence base that supports physiology-guided revascularisation largely excluded the LMCA, due to complex anatomical and physiological considerations, such as downstream serial disease. We have suggested a simple algorithm involving FFR pullback manoeuvres from both the LAD and LCx branches, with aid from intravascular imaging if further clarification is needed in equivocal cases, that will provide diagnostic clarity in most scenarios. Whilst non-randomised data suggest that physiology-guided management is valid and equally effective for the LMCA, we await randomised controlled trial data to support its widespread use. |
Funding
B. Modi is funded by a British Heart Foundation Clinical Research Training Fellowship (FS/15/78/31678).
Conflict of interest statement
The authors have no conflicts of interest to declare.

