Abstract
Aims: The study sought to assess the effect of percutaneous pulmonary artery denervation (PADN) on balloon-occlusion-induced acute pulmonary arterial hypertension (PAH) in vivo. The PADN is a minimally invasive and endovascular catheter-based interventional therapy using radiofrequency ablation to abolish the pulmonary arterial baroreceptors to pressure response.
Methods and results: To examine the efficacy of balloon-occlusion-induced PAH, twenty Mongolian dogs were randomly assigned to one of two groups: group 1 (left distal pulmonary basal trunk occlusion) and group 2 (left pulmonary interlobar artery occlusion). Afterwards, PADN treatment at the main pulmonary artery bifurcation level with left pulmonary interlobar artery occlusion in all 20 dogs was conducted. Haemodynamic parameters were measured at baseline and during balloon occlusion as well as the PADN treatment at different time points: one, two, three, five, and ten minutes. Before the PADN treatment, most haemodynamic parameters of the pulmonary artery remained unchanged in group 1 with distal pulmonary basal trunk occlusion. However, in group 2 with the occlusion of the left pulmonary interlobar artery, mean pulmonary arterial pressure, mean right ventricular pressure, and pulmonary vessel resistance gradually increased, and mean absolute difference reached peak at five minutes (Δ16.6 mmHg, Δ14.1 mmHg and Δ1,144 dye/s/cm5, respectively; each p<0.01). These haemodynamic parameters at five minutes induced by left pulmonary interlobar artery occlusion were completely abolished with the PADN treatment compared to baseline (Δ0.3 mmHg, Δ0.2 mmHg, and Δ34 dye/s/cm5, respectively).
Conclusions: Balloon occlusion of the left pulmonary interlobar artery led to a significant increase of haemodynamic parameters of the pulmonary artery. The pressure responses were completely abolished by the PADN treatment at the main bifurcation area of the left pulmonary artery.
Introduction
Pulmonary arterial hypertension is defined as a group of diseases characterised by a progressive increase in pulmonary vascular resistance resulting in right heart failure and premature death1-7. Recent therapeutic advances have improved treatment options including prostanoids, endothelin-receptor antagonists and the phosphodiesterase type-5 inhibitors3-6,8,9. A meta-analysis of 23 randomised controlled trials demonstrated a reduction in mortality of the patients using the targeted therapies approved for pulmonary arterial hypertension (PAH)10. However, there is no cure for PAH and it remains a life-threatening disorder even though these therapies improve symptoms, haemodynamics and outcomes11.
The pathogenesis of PAH is complicated and still largely unknown. In particular, the role of sympathetic nerve activation remains unsettled12. Historically, several studies have demonstrated that distention and occlusion of one branch of the pulmonary artery by the use of balloons and non-occlusive inflatable cuffed cylinders led to significant increases of pulmonary arterial pressure (PAP) and pulmonary vessel resistance (PVR)13-18. Of note, the increase of PAP and PVR induced by pulmo-pulmonary reflex was confirmed. To clarify the underlying mechanisms involved in this procedure, experimental study showed that pulmonary hypertension may be secondary to the distention of the main pulmonary artery by excitation of stretch receptors in or near the bifurcation area of the main pulmonary artery. In addition, the efferent limb of this reflex was predominantly mediated by the adrenergic nervous system19. Based on the evidence mentioned above, we hypothesised that percutaneous catheter-based pulmonary artery denervation (PADN) around the bifurcation area of the main pulmonary artery by radiofrequency ablation might abolish the baroreceptors or sympathetic nervous fibres of the pulmonary artery. Thus, it can be predicted that balloon-occlusion-induced pulmonary arterial pressure increase will be blunted.
Methods
STUDY DESIGN
The study design is shown in Figure 1. Briefly, twenty Mongolian dogs were randomly assigned to one of two groups: group 1 (left distal pulmonary basal trunk occlusion), and group 2 (left pulmonary interlobar artery occlusion). The occlusion model was conducted by the use of a balloon to stop the distal blood flow completely. Haemodynamic parameters were measured before and after balloon occlusion using a Swan-Ganz pulmonary artery catheter (Baxter Healthcare Corp., Irvine, CA, USA). The effect of PADN treatment at the main pulmonary artery bifurcation level was investigated by repeating the above-mentioned procedure followed by left pulmonary interlobar artery occlusion. The study was approved by the Nanjing First Hospital IRB committee (#NJMEDHOS 76895211) and followed the National Research Council for animal research guidelines.
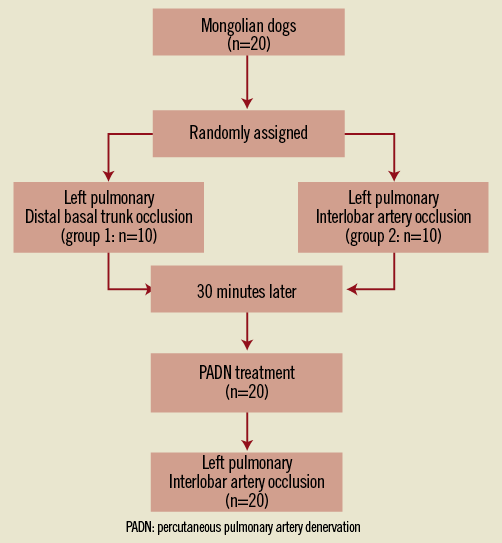
Figure 1. Study design. PADN: percutaneous pulmonary artery denervation.
UNILATERAL PULMONARY ARTERY OCCLUSION AND HAEMODYNAMIC MEASUREMENTS
Sequential unilateral left pulmonary artery occlusion was performed. Briefly, a 6 Fr guiding catheter was engaged in the left pulmonary artery and a pulmonary arteriography was performed (Figure 2A). A balloon with a diameter of 2.5 mm (8 mm in length) was positioned in the distal pulmonary basal trunk and was inflated at 16 atm for ten minutes in group 1 (Figure 2B). In group 2, a balloon with a diameter of 5 mm (8 mm in length) was advanced to the pulmonary interlobar artery, and was inflated at 16 atm for ten minutes (Figure 2C). Pulmonary arteriography was performed to confirm the total occlusion of blood flow distal to the balloon. Haemodynamic measurements were performed before, one, two, three, five, and ten minutes after balloon occlusion.
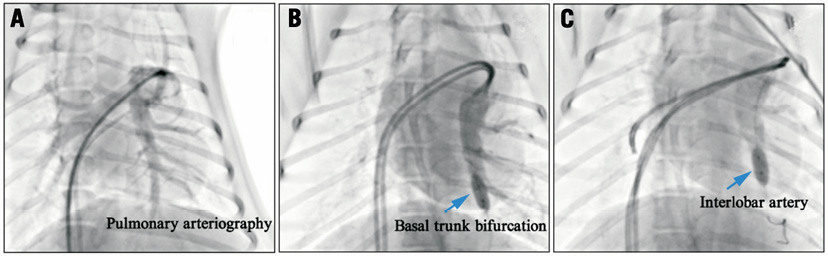
Figure 2. Pulmonary arteriography of the animal model. A) Pulmonary arteriography showed the basal trunk and the interlobar artery. B) A balloon at the left distal pulmonary basal trunk bifurcation level. C) A balloon at the left pulmonary interlobar artery occluded distal blood flow. It is more proximally located than the basal trunk.
A 7 Fr flow-directed Swan-Ganz catheter (Baxter Healthcare Corp.) was inserted percutaneously via the femoral vein for the measurement of resting right atrial pressure (RAP), right ventricular pressure (RVP), systolic/diastolic and mean PAP, pulmonary artery occlusion pressure (PAOP), thermodilution cardiac output (CO), and mixed venous oxygen saturation. PVR=(mean PAP-PAOP)/CO and transpulmonary gradient (TPG)=mean PAP-PAOP were then calculated20-22.
DEDICATED APPARATUS FOR PULMONARY ARTERY HYPERTENSION
A dedicated 7.5 Fr triple-function (mapping, temperature-sensing, and ablation) catheter (PADN™; MicroPort Co., Shanghai, China) along an 8 Fr long sheath was advanced beyond the pulmonary valvular level. This catheter has a tapered (to 5 Fr) circular tip with 10 electrodes (each with a width of 0.75 mm and separated by 2 mm, Figure 3A) pre-mounted. The circular tip consists of a self-adjustable inner ring (with diameters of 15 mm, 17 mm, 20 mm, and 22 mm) as well as an outer ring (with diameters of 25 mm, 28 mm, 30 mm and 33 mm, respectively). The position and direction of rings are adjusted by manipulating a rotating handle (Figure 3B). There are 10 manual knobs on the surface of the radiofrequency generator, each connected to a specific electrode on the rings (Figure 3C).
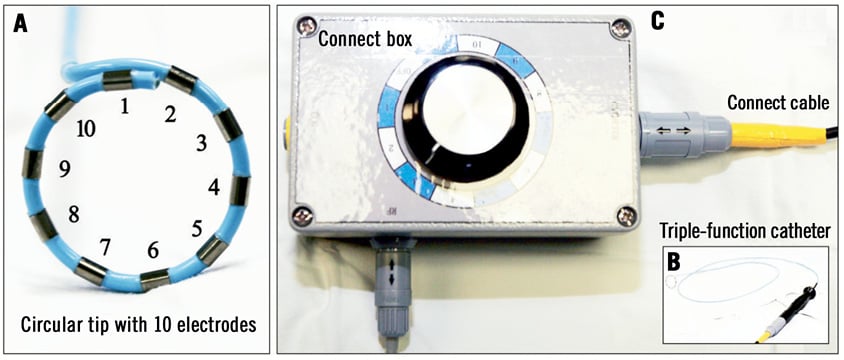
Figure 3. A dedicated radiofrequency percutaneous catheter-based pulmonary artery denervation system. A) A dedicated 7.5 Fr triple-function catheter had a tapered (to 5 Fr) circular tip with 10 electrodes (each has 0.75 mm electrode-width and is separated by 2 mm) pre-mounted. B) The circular tip consists of an inner ring and an outer ring, on which electrodes are connected to the generator. C) Sequential ablations were performed by selecting the knob on the generator.
PULMONARY ARTERY DENERVATION PROCEDURE
Based on the anatomy of pulmonary arteriography23, one of five different ablation levels was potentially selected as a candidate for PADN treatment (Figure 4): Level 1 (<1 mm distal to orifice of left branch), Level 2 (<1 mm distal to ostial right branch), Level 3 (<2 mm proximal to bifurcation level), Level 4 (2 mm to 5 mm distal to ostial right branch), and Level 5 (2 mm to 5 mm distal to ostial left branch). Preliminary experiments in our lab showed that Level 3 was the most effective area for PADN treatment (data not shown). In addition, PADN treatment at level 4 and 5 in sites 2 mm to 5 mm distal to the ostial right or left pulmonary artery failed to abolish the pressure responses to balloon inflation at the left pulmonary interlobar artery.
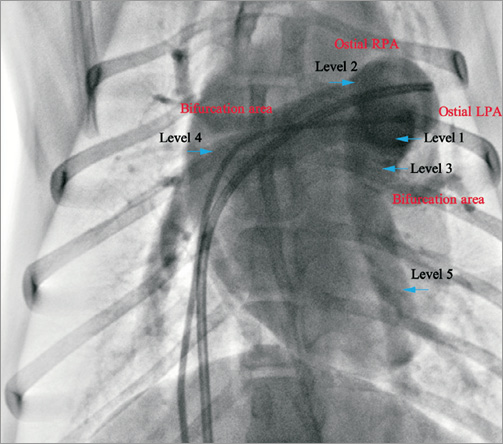
Figure 4. Different radiofrequency ablation levels of the animal model. Five ablation levels were performed at Level 1 (<1 mm distal to orifice of left branch), Level 2 (<1 mm distal to ostial right branch), Level 3 (<2 mm proximal to bifurcation level), Level 4 (2-5 mm distal to ostial right branch) or Level 5 (2-5 mm distal to ostial left branch).
In the present study, after haemodynamic parameters returned to baseline levels, all dogs in both groups (n=20) received the PADN treatment at Level 319. To ensure that the electrodes are tightly contacting the arterial inner surface, three criteria are used as follows: (1) stronger resistance felt when rotating the handle, (2) inability to advance distally or ease of withdrawing proximally, and (3) angiography confirmation. After the first round of ablation, the catheter tip is withdrawn and rotated and re-advanced to ensure the whole circular axis of the vessel is ablated. The ablation parameters at each point were programmed as: temperature 50°C, energy ≤10 W, impedance <140 Ω, time 20 sec.
STATISTICAL ANALYSIS
All data are expressed as the mean ± SEM of results obtained from the number of experiments. Normal distribution was checked by the Kolmogorov-Smirnov test. Differences between data sets were assessed by paired t-tests or the Wilcoxon rank-sum test. All analyses were undertaken using SPSS 20.0 (IBM Corp., Armonk, NY, USA). A probability value of <0.05 was considered statistically significant (two-sided).
Results
PULMONARY ARTERIAL PRESSURE RESPONSE TO LEFT DISTAL PULMONARY BASAL TRUNK OCCLUSION BY BALLOON INFLATION BEFORE PADN (GROUP 1)
Baseline systolic PAP, mean PAP, and diastolic PAP were 25.7±1.6 mmHg, 14.1±0.7 mmHg, and 8.5±1.2 mmHg, respectively. After balloon occlusion of the left distal pulmonary basal trunk by inflation with a diameter of 2.5 mm, only at three minutes during occlusion, the pulmonary arterial parameters increased statistically compared to the baseline, but the absolute differences were numerical (4.3 mmHg for SPAP, 1.8 mmHg for MPAP and 1.2 mmHg for SRVP, Figure 5).
During continuous occlusion of the left distal pulmonary basal trunk, pulmonary artery occlusion pressure (PAOP), cardiac output (CO), pulmonary vascular resistance (PVR), and heart rate remained stable without significant changes before and during balloon inflation (Table 1).

PULMONARY ARTERIAL PRESSURE RESPONSE TO LEFT PULMONARY INTERLOBAR ARTERY OCCLUSION BY BALLOON INFLATION BEFORE PADN (GROUP 2)
Baseline systolic PAP, mean PAP, and diastolic PAP were 23.4±0.9 mmHg, 13.9±0.5 mmHg, and 9.6±0.6 mmHg, respectively. During occlusion of the left pulmonary interlobar artery by balloon inflation of a diameter of 5 mm, mean PAP gradually increased and reached peak value (30.5±1.1 mmHg) at five minutes, with absolute difference of 16.6 mmHg. Between five and ten minutes, increased pulmonary arterial pressure persisted (Figure 6).
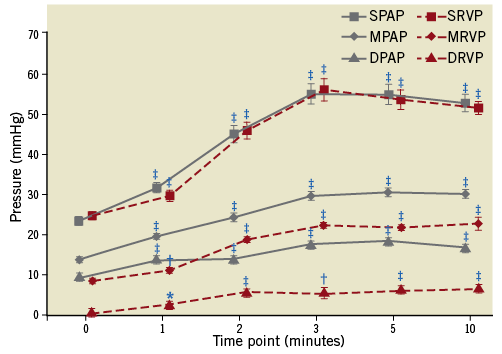
Figure 6. Changes of haemodynamics before and during balloon occlusion of the left pulmonary interlobar artery. *indicated p<0.05, compared with baseline (before occlusion); †indicated p<0.01, compared with baseline; ‡indicated p<0.001, compared with baseline. DPAP: diastolic pulmonary arterial pressure; DRVP: diastolic right ventricular pressure; MPAP: mean pulmonary arterial pressure; MRVP: mean right ventricular pressure; SPAP: systolic pulmonary arterial pressure; SRVP: systolic right ventricular pressure
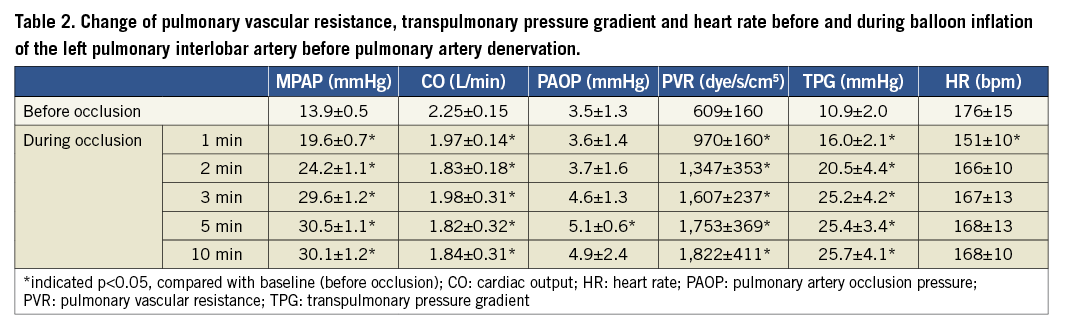
Notably, because there were no significant increases of CO and PAOP, we detected that a significant increment of mean PAP resulted in the higher PVR up to three minutes (1,607±237 dye/s/cm5), five minutes (1,753±369 dye/s/cm5) and ten minutes (1,822±411 dye/s/cm5), when compared to baseline PVR (609±160 dye/s/cm5, all p<0.01, Table 2).
PULMONARY ARTERIAL PRESSURE RESPONSE TO LEFT PULMONARY INTERLOBAR ARTERY OCCLUSION BY BALLOON INFLATION AFTER PADN
There were no adverse outcomes and relative complications (death, perforation, acute thrombus formation) pre and post the PADN procedure. The average procedural time was 18.6 minutes.
Immediately after the PADN procedure, systolic PAP, mean PAP, and diastolic PAP were 29.8±1.7 mmHg, 16.9±1.5 mmHg, and 12.8±1.7 mmHg, respectively. The peak systolic PAP, mean PAP, and diastolic PAP were observed at three minutes after balloon occlusion. At the five and ten minute time points, there was no significant difference in all of the haemodynamic parameters observed (Figure 7). In addition, there was no significant change of PVR between baseline and five minutes (569±330 dye/s/cm5 vs. 603±258 dye/s/cm5, p=0.601). Similarly, CO, PAOP and heart rate did not change significantly (Table 3).
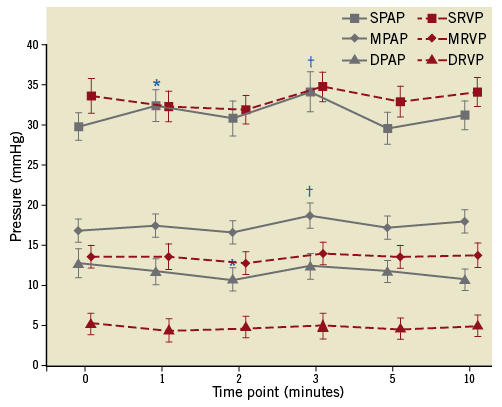
Figure 7. Changes of haemodynamics before and during balloon occlusion of the left pulmonary interlobar artery after pulmonary artery denervation in the main pulmonary artery bifurcation area. *indicated p<0.05, compared with baseline (before occlusion); †indicated p<0.01, compared with baseline. DPAP: diastolic pulmonary arterial pressure; DRVP: diastolic right ventricular pressure; MPAP: mean pulmonary arterial pressure; MRVP: mean right ventricular pressure; SPAP: systolic pulmonary arterial pressure; SRVP: systolic right ventricular pressure

Discussion
We hypothesised that experimental pulmonary hypertension can be caused by a reflex mechanism from balloon occlusion of the pulmonary artery. From this experimental study, we reported for the first time that: (1) occlusion of the left pulmonary interlobar artery rather than the left pulmonary basal trunk by balloon inflation induced significant increases of PAP and RVP; (2) PADN at the main bifurcation area of the pulmonary artery using a dedicated ablation catheter might completely abolish the pulmonary artery pressure response to the inflated balloon occlusion.
ACUTE PULMONARY ARTERIAL HYPERTENSION MODEL
According to previous experimental animal models, unilateral pulmonary arterial distention was mainly produced by stepwise inflation of a balloon or by non-occlusive inflatable cuffed cylinders13-19.
However, controversy existed about the mechanisms resulting in a significant increase of PAP or PVR. Several studies have shown that the baroreceptors in the pulmonary artery branches contributed to the mediation of PAP but without evidence of stimulation of vagal nerves when blood flow was totally or sub-totally occluded13,14. However, Ueda et al did not find direct evidence of the sympathetic nerves in this pulmo-pulmonary reflex15. In another study, Murao et al postulated that this pressure response was solely a mechanical effect involving vasoconstriction16. Regardless of the mechanism, the use of the non-occlusive (triple-lumen) balloon distention (stepwise inflation) of the main pulmonary artery at the bifurcation area induced the most significant increases in PAP and PVR18,19. The present study is consistent with previous findings that pulmonary arterial pressure increased significantly using balloon occlusion of the pulmonary artery. This finding represented the fact that acute pulmonary arterial hypertension can be reproduced experimentally and thus it is important to validate the efficacy of the PADN treatment in our study.
BARORECEPTORS AND PULMONARY ARTERIAL HYPERTENSION
Baroreceptors are sensors located in the blood vessels of several mammals: they detect blood pressure and send messages to the central nervous system to increase resistance when necessary24. Baroreceptors can be classified as arterial (high-pressure) or venous (low-pressure)25. Accumulative evidence implied that the baroreceptors might lie in the main pulmonary artery, probably in or near the bifurcation rather than in the branches themselves. This postulation was examined by Juratsch and colleagues demonstrating that surgical dissection of the areas of the pulmonary artery under the bifurcation and adjacent to the aorta were particularly necessary to abolish the pressure response by balloon inflations19. In addition, the sympathetic nerves potentially providing both the afferent and efferent pathways for this reflex were investigated14,18,19. Efferent sympathetic nerve endings participating in the rise of pressure have been examined by both animal and clinical studies18,19,26-30. On the other hand, afferent sympathetic activity originating from the pulmonary artery has been found in the 3rd and 4th rami of the spinal cord31-33.
PADN TREATMENT
The sites of the sympathetic nerve endings can also be postulated from our study. A previous study reported that the low-pressure baroreceptors are buried in the pulmonary arterial wall close to the intima19. However, the high-pressure baroreceptor sensory endings mostly lie in the tunica adventitia of the artery. Thus, it is crucial to remove the adventitia and some portions of the medial layers in order to abolish these baroreceptors. In the present study, PADN treatment completely abolished the pressure response to balloon inflations. Such pressure response, however, was not abolished when PADN was performed in the sites distal (2 mm to 5 mm) to the bifurcation level of the pulmonary artery. This may suggest that the sympathetic nerve endings were localised in or near the area of the bifurcation level of the pulmonary artery.
With the development of sympathetic denervation for the treatment of refractory systemic hypertension by renal ablation denervation technology34-36, we recognised the potential of pulmonary artery denervation for the treatment of pulmonary hypertension. From our experience, there are several limitations of the conventional catheter when used for PADN including: the difficulty of contacting the vessel’s inner surfaces tightly, the need for multiple rotations to complete circular ablation, and the high risk of perforation. Thus, we designed a special ablation catheter with a circular tip for this study, where only slight movements were required to keep tight contact with the vessel. As a result, we anticipate that pulmonary artery denervation therapy could be performed safely and successfully.
Clinical implications
Although medical therapy for PAH was evaluated extensively, the chronic treatment of PAH remains unsatisfactory. Symptomatic improvement is not always possible. Once the diagnosis of pulmonary hypertension is established, the prognosis remains poor. As a result, this novel PADN treatment in the present study may provide new hope for therapeutics in patients with pulmonary arterial hypertension.
Limitations
The study has several limitations. First, the balloon occlusion was not inflated in a stepwise manner. It would have induced vasoconstriction of the pulmonary artery13-17, leading to a rise in both PAP and systolic right ventricular pressure. However, this present study was to examine the effect of PADN on PAP, and did not focus on the mechanisms in the changes of haemodynamics during balloon inflation. Second, the balloon occlusion was repeated after PADN therapy in group 1 and group 2. The tolerance effect of the pulmonary haemodynamics parameters from repeated balloon occlusions was not assessed. Third, there was an absence of histological evidence from the PADN treatment in our study. The lack of histological proof of sympathetic nerve damage weakened our hypothesis of damaged nerve endings by our ablation techniques. However, the safety of the PADN treatment was approved, as no experimental dogs died after the PADN treatment. Finally, the current study only reported that PADN treatment abolished the acute experimental pulmonary hypertension. The effect of PADN treatment in chronic pulmonary hypertension or irreversible condition remains unsettled. Further experimental studies will address the details of precise mechanisms and different settings with respect to the PADN treatment. As a result of the safety and efficacy of PADN treatment in the present study, a FIM study is expected to be organised in the near future.
Conclusions
We are the first to report that pulmonary artery denervation in the main pulmonary artery bifurcation area with our newly designed system might completely abolish the experimental pulmonary hypertension response to balloon inflations.
Acknowledgements
We appreciated the efforts of Ms. Tian Xu and Ms. Rong Wang for their data collection.
Conflict of interest statement
The authors have no conflict of interest to declare.

