Abstract
Pulmonary arterial hypertension (PAH) is a group of diseases related to progressively increasing pulmonary vascular resistance, a high incidence of right ventricular failure and premature death. Only a limited number of pharmaceutical therapies have proven to be beneficial for PAH. These therapies improve symptoms, exercise capacity, and haemodynamics; however, the clinical relevance of these effects has been challenged. Therefore, the effect of currently approved treatment options remains inconclusive. Conversely, several new drugs for various aetiologies and clinical stages are expected to provide significant advances for the treatment of PAH. Moreover, percutaneous pulmonary artery denervation treatment may lead to a new therapeutic orientation in patients with PAH. The aim of this review is to present the new developments in PAH treatment, provide a brief overview of future directions in the field and discuss the potential future prospects of these innovative therapies.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive condition of multifactorial aetiology, which is clearly associated with a poor prognosis and results in right ventricular failure and premature death1,2. It includes idiopathic PAH (IPAH) and PAH related to various conditions such as congenital systemic-to-pulmonary shunts, collagen vascular disease, portal hypertension, anorexigen use, and human immunodeficiency virus infection3,4.
Currently approved therapies for the treatment of PAH include prostanoids, endothelin-receptor antagonists, and the phosphodiesterase type 5 inhibitors5. Conversely, guidelines and expert consensus documents containing assistance on selecting the best management for pulmonary hypertension (PH) patients also recommend the use of supportive measures, such as diuretics, oral anticoagulants, long-term oxygen therapy and digoxin as indicated4,6. Although these agents are efficacious in improving clinical symptoms, exercise capacity and haemodynamics, the clinical significance of these effects and their prognostic relevance has been challenged7-9. Currently, the treatment of PAH is based on limited understanding of the disease pathogenesis, and its underlying mechanisms remain largely unknown.
Of note, a previous experimental study showed that surgical dissection of the areas of the pulmonary artery under the bifurcation and adjacent to the aorta were necessary to abolish the pressure response to balloon inflations10. In addition, the efferent limb of this reflex was predominantly mediated by the adrenergic nervous system. Recently, an acute animal study based on this mechanism reported that the increased haemodynamic parameters during the balloon occlusion of the left pulmonary interlobar artery were completely abolished by percutaneous pulmonary artery denervation (PADN) treatment11. Although the safety and efficacy of this novel PADN treatment for patients with PAH has yet to be established, it may provide a new therapeutic avenue for this disease.
Over the last several decades, considerable efforts have been made to develop new therapeutics for PAH. In this review article, we present the most recent developments in PAH treatment, provide a brief overview of future directions in the field, and discuss the potential future prospects of these innovative therapies.
Pharmacologic therapies for PAH
Currently, there are more than seven drugs with different routes of administration available (Table 1) for the treatment of PAH, most of which lead to a significant improvement in patients’ symptoms and a slower prevalence of clinical deterioration. A meta-analysis of 23 randomised controlled trials (RCTs) in patients with PAH showed a reduction of overall mortality (38%) in patients randomised to active treatments compared to those randomised to placebo control arms12. The beneficial effects of these drugs were confirmed, even with the inclusion of RCTs on compounds which were eventually not approved by the regulatory agencies due to lack of consistent efficacy13-15. The following sections describe primarily the specific drugs that are commercially available, or currently under clinical evaluation, and present the evidence from their application in clinical settings.
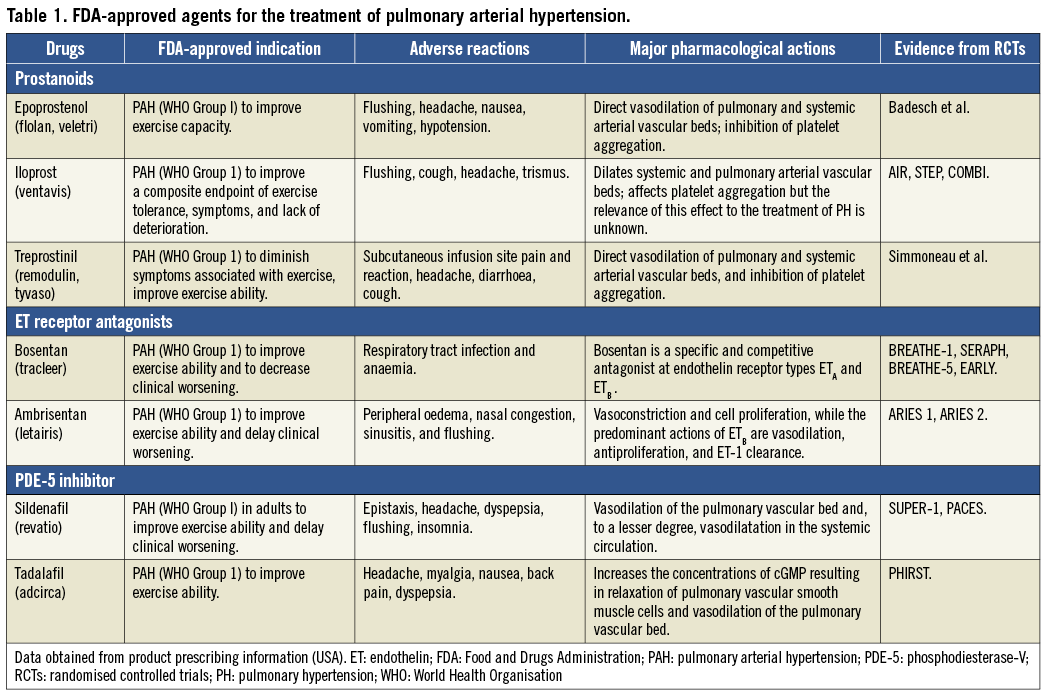
PROSTANOIDS
Prostacyclin is formed primarily by endothelial cells and induces potent vasodilatation of vascular beds, which can also inhibit platelet aggregation and have both cytoprotective and antiproliferative activities16. Epoprostenol is a synthetic analogue of prostacyclin, which has been extensively evaluated in clinical trials. Epoprostenol with a short half-life (<5 mins) and limited stable time at room temperature (<8 hours) requires to be administered by an infusion pump or a permanent tunnelled catheter. The efficacy of continuous infusion of epoprostenol was evaluated in several randomised controlled trials. Although continuous i.v. administration of epoprostenol attenuates symptomatic deterioration and improves exercise capacity and haemodynamic status, increased survival rate was only observed in idiopathic/heritable PAH. Conversely, the adverse effect of accelerated hypoxia caused by epoprostenol treatment is a concern, especially in patients with serious chronic lung diseases. It was more likely to be associated with increased shunt flow in the lung, resulting in the development of ventilation-perfusion mismatch and hypoxia in patients with lung fibrosis. The clinical effects of other prostacyclin derivatives (namely, iloprost and treprostinil) are similar to epoprostenol. The routine use of these prostacyclin derivatives in patients with PAH has been extended and recommended (WHO III/IV)4,17.
ENDOTHELIN RECEPTOR ANTAGONISTS
Patients with PAH have been shown to be associated with activation of the endothelin system in both plasma and lung tissue18. However, it is still unknown whether increased endothelin-1 (ET-1) expression is a cause or a consequence of PAH19. The biologic effects of ET-1 are regulated primarily by two distinct receptors, ETA and ETB. Although it is controversial whether selective ETA blockade or dual ETA/ETB blockade is the best approach for patients with PAH, the efficacy of these drugs appears to be comparable. Recent studies have suggested that the most therapeutic effect is brought about by the blockade of both receptors20-23. In an experimental study comparing the roles of ETA and ETB receptors in the pathogenesis of monocrotaline-induced PH, the haemodynamic and histologic parameters were improved due to ETA receptor antagonism24. It is reasonable to assume that these drugs lead to comparable pharmacologic and therapeutic effects in haemodynamics, right ventricular hypertrophy, pulmonary arterial remodelling, pulmonary vessel endothelial function and survival treated by either selective or non-selective ET receptor antagonists25.
Currently, there are two ET receptor antagonists approved as first-line use in patients with mild to moderate PAH: bosentan and ambrisentan. Bosentan is an oral active dual ETA and ETB receptor antagonist. It has been tested in several RCTs for treating PAH patients, showing that there was significant improvement in exercise capacity, functional class, haemodynamics, and even timing to clinical deterioration26-30. The clinical activity of ambrisentan is similar to that of bosentan. Although hepatic injury was mild with ambrisentan administration31, bosentan led to adverse effects on the liver with a frequency of approximately 10%30.
PHOSPHODIESTERASE TYPE 5 INHIBITORS
A number of randomised controlled studies have reported favourable effects of the orally active phosphodiesterase-V (PDE-5) inhibitor (namely, sildenafil and tadalafil) in patients with PAH32-35. The effect was related to vasodilation via increasing cGMP concentrations, inhibiting voltage-gated calcium channels36. These trials have demonstrated that PDE-5 inhibitors improved exercise capacity, haemodynamic status and clinical events34,37. The side effects (headache, flushing and epistaxis, etc.) of PDE-5 inhibitors were mild to moderate and largely associated with vasodilation.
COMBINATION THERAPY
Combination therapy is a prospective option in addressing the multiple pathophysiologic mechanisms that are present in PAH. The safety and efficacy of combination therapy have been investigated in several small studies30,38-40. Although the optimal combination on the basis of overall risk-benefit considerations remains undefined, these preliminary studies supported the use of co-treatments in order to achieve the best possible clinical outcomes. Recently, a meta-analysis suggested that combination therapy does not offer an advantage over monotherapy in treating patients with PAH41. Given the paucity of good quality data, the conclusions from the meta-analysis of observational studies were poor. Therefore, further research with randomised controlled trials should be performed to provide more confirmative information before establishing final guidelines. In patients with PAH who are deteriorating despite chronic treatment with non-parenteral prostanoids, the addition of bosentan or sildenafil to the ongoing treatment resulted in favourable improvements of pulmonary haemodynamics and exercise capacity in uncontrolled studies39,40.
NEW DRUGS
As mentioned previously, the progress in treatments for PAH, together with the functional improvements and the survival rate of PAH patients, remain unsatisfactory. In addition, many insurance companies do not reimburse patients for the costs of drug administration, and the side effects after these pharmacologic therapies have become apparent. For these reasons, new drugs (namely, imatinib, riociguat, selexipag and macitentan, etc.) with low incidences of side effects are expected to improve symptoms and prognosis42. For example, riociguat is a novel, first-in-class oral drug that directly stimulates soluble guanylate cyclase, both independently of the endogenous vasodilator nitric oxide (NO) and in synergy with NO43. Recently, the CHEST-1 trial which enrolled 263 patients of CTEPH showed that riociguat treatment significantly improved six-minute walking distance from baseline compared with placebo (46 m, 95% CI: 25-67 m, p<0.001), PVR (p<0.001), NT-proBNP (p<0.001), and WHO function class (p=0.0026)44.
Percutaneous pulmonary artery denervation treatment for PAH
Historically, Juratsch et al demonstrated that surgical dissection of pulmonary artery bifurcation areas was more likely to abolish the pressure response to balloon inflations10. Moreover, sympathetic nerves providing both the afferent and efferent pathways for this reflex were investigated, showing that efferent sympathetic nerve endings participate in the rise of pressure45,46. Based on the above-mentioned evidence, a new alternative to current pharmacotherapy might provide hope for patients with PAH.
EXPERIMENTAL STUDY
Recently, our group for the first time reported that percutaneous catheter-based pulmonary artery denervation (PADN) situated around the bifurcation area of the main pulmonary artery by radiofrequency ablation completely abolished the baroreceptors or sympathetic nervous fibres of the pulmonary artery (Figure 1). Although the haemodynamic parameters were significantly increased after three minutes of left pulmonary interlobar artery occlusion by balloon inflation, immediately after the PADN procedure systolic PAP, mean PAP, and diastolic PAP were only 29.8±1.7 mmHg, 16.9±1.5 mmHg, and 12.8±1.7 mmHg, respectively. Compared to the values without PADN treatment, all the increased haemodynamic characteristics were significantly attenuated. This specific treatment relied upon a novel dedicated apparatus. The denervation system applied in the experimental study comprised three major elements (Figure 2): a dedicated 7.5 Fr triple-function catheter with 10 pre-mounted electrodes (each had 0.75 mm electrode width and was separated by 2 mm), a circular tip consisting of an inner ring and an outer ring, and a generator with sequential ablations administered by the console.
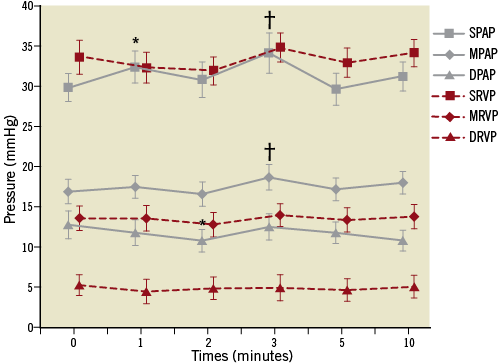
Figure 1. Changes of haemodynamics11. Increased haemodynamic parameters induced by balloon occlusion of the left pulmonary interlobar artery were almost abolished by percutaneous pulmonary artery denervation treatment around the bifurcation area of the main pulmonary artery. *indicates p<0.05, compared with baseline (before occlusion); †indicates p<0.01, compared with baseline. DPAP: diastolic pulmonary arterial pressure; DRVP: diastolic right ventricular pressure; MPAP: mean pulmonary arterial pressure; MRVP: mean right ventricular pressure; SPAP: systolic pulmonary arterial pressure; SRVP: systolic right ventricular pressure
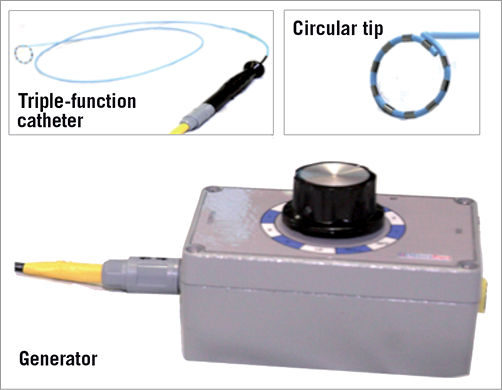
Figure 2. Dedicated radiofrequency apparatus for pulmonary artery denervation treatment. The dedicated denervation system comprises three major parts as follows: 1) a dedicated 7.5 Fr triple-function catheter with 10 electrodes, 2) a circular tip consisting of an inner ring, and 3) an outer ring, a generator with sequential ablations by selecting the knob.
FIRST-IN-MAN STUDY
A prospective first-in-man (FIM) PADN-1 study from our group reported at TCT 2012 that PADN treatment for patients with PAH is safe and feasible. This percutaneous invasive treatment improved functional capacity and haemodynamic parameters in patients who failed to respond to standard medications47. The average improvement of functional capacity using the six-minute walk test in a meta-analysis was 35.6 m (–10 to +108)12. Conversely, the PADN-1 study showed an absolute increase of 101 m at three months after the PADN treatment.
In the FIM study, three levels (level 1: <2 mm distal to orifice of left PA; level 2: <2 mm proximal to bifurcation level; level 3: <2 mm distal to ostial right PA) of pulmonary artery were selected for PADN treatment. The procedure of PADN treatment for patients with PAH is briefly described as follows: 1) the dedicated ablation catheter was advanced through an 8 Fr long sheath; 2) the circular tip with 10 electrodes was released after withdrawing the sheath and pushing the catheter; 3) the three levels of pulmonary artery were ablated with our dedicated catheter. During the ablation, the electrodes were confirmed to be tightly in contact with the endovascular surface. The following ablation parameters were programmed at each level: temperature >50°C, energy 10 W and time 60 sec. The electrocardiogram and haemodynamic parameters were examined and continuously recorded during the procedure. Procedural success was defined as a reduction in the mean pulmonary arterial pressure ≥10 mmHg. The patients remained in the coronary care unit for at least 24 hours after the procedure.
With respect to procedural safety, there were no adverse outcomes or relative complications (death, perforation and acute thrombus formation, etc.) pre- and post-PADN treatment in the PADN-1 study. In fact, the potential risks in terms of PADN treatment may include femoral artery pseudoaneurysm and pulmonary artery dissection related to catheter manipulation. In addition, the safety and efficacy of using this novel invasive strategy for patients with PAH should be investigated in the future. Apart from this FIM study, the randomised controlled trial (PADN-2) aiming to estimate the short- and long-term haemodynamic parameters and clinical outcomes of PADN treatment and pharmacotherapy is currently ongoing (Registration number: ChiCTR-TRC-12002097).
Conclusions
In the field of PAH, pharmacologic therapies have recently been developed. Concurrently, new guidelines for the treatment of patients with PAH have been published4. The choice of drugs should be dependent on several variables, such as the route of administration, the approval status, the side-effect profile, the patient’s preference, the physician’s experience and clinical judgement. Additional therapeutic strategies targeted to diverse pathophysiological changes are being explored in order to improve symptoms and clinical prognosis. Although combination therapy might be considered for patients whose clinical symptoms fail to be improved or even deteriorate with monotherapy, many unanswered questions remain, particularly related to the choice of combination drugs. Moreover, the interactions involving the disease-specific therapies for PAH should be avoided to prevent adverse complications. In addition, new drugs are becoming available for clinical use and more research is warranted.
Atrial septostomy and lung transplantation are optional palliative therapeutics for patients with severe PAH who have significant right ventricular failure, symptoms which are refractory to maximal medical therapy. However, patients should be carefully selected, especially in these cases where pharmacologic therapies are not seen to be effective. Although there is no cure for this progressive disease, given the promising early experience of PADN treatment it may prove to become an efficacious alternative treatment strategy for PAH patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

