
Abstract
Aims: Pulmonary arterial hypertension is a devastating disease characterised by pulmonary vascular remodelling and right heart failure. Radio-frequency pulmonary artery denervation (PDN) has improved pulmonary haemodynamics in preclinical and early clinical studies; however, denervation depth is limited. High-frequency non-focused ultrasound can deliver energy to the vessel adventitia, sparing the intima and media. We therefore aimed to investigate the feasibility, safety and efficacy of ultrasound PDN.
Methods and results: Histological examination demonstrated that innervation of human pulmonary arteries is predominantly sympathetic (71%), with >40% of nerves at a depth of >4 mm. Finite element analysis of ultrasound energy distribution and ex vivo studies demonstrated generation of temperatures >47ºC to a depth of 10 mm. In domestic swine, PDN reduced mean pulmonary artery pressure induced by thromboxane A2 in comparison to sham. No adverse events were observed up to 95 days. Histological examination identified structural and immunohistological changes of nerves in PDN-treated animals, with sparing of the intima and media and reduced tyrosine hydroxylase staining 95 days post procedure, indicating persistent alteration of the structure of sympathetic nerves.
Conclusions: Ultrasound PDN is safe and effective in the preclinical setting, with energy delivery to a depth that would permit targeting sympathetic nerves in humans.
Introduction
Pulmonary arterial hypertension (PAH) is a complex disorder characterised by increased pulmonary vascular resistance and right heart failure. Current therapies provide benefit through modulation of the prostacyclin, endothelin-1 and nitric oxide pathways1; however, patients continue to experience significant morbidity and mortality2.
Sympathetic and neurohormonal activity including plasma norepinephrine3,4, muscle sympathetic nerve activity5,6 and indicators of renin–angiotensin–aldosterone system7 activity are increased in patients with PAH and are associated with adverse clinical outcome4,5,6. Modulation of the sympathetic and renin–angiotensin–aldosterone systems is an established treatment for heart failure8,9,10,11,12,13. Both beta-blockers14,15 and angiotensin-converting enzyme inhibitors7 have been shown to attenuate disease in experimental models; however, there is no evidence of benefit in patients with PAH16,17,18.
Preclinical studies have demonstrated that pulmonary artery denervation (PDN) improves pulmonary haemodynamics in acute19,20 and chronic models of pulmonary hypertension (PH)21 and in a single-centre study of patients with PAH22. A single case report used high-output burst electric stimulation applied within the pulmonary artery to induce autonomic responses23; however, the distribution of nerves around the pulmonary arteries in humans remains largely unknown. We therefore examined nerve distribution around the pulmonary arteries in humans and, using a novel ultrasound energy delivery system, we sought to demonstrate histological evidence of a thermal effect on pulmonary artery adventitial nerves and a haemodynamic effect in an acute vasoconstrictive model of PH.
Methods
HUMAN PULMONARY ARTERY NERVE DISTRIBUTION
Human cadavers and histological samples were examined to determine the distribution of pulmonary artery innervation.
CADAVERIC
Anatomical examination was conducted at the Vienna Medical University, Austria, in accordance with European Commission (EC) approval (1424/2015). The rib cage was lifted to allow inspection of the thoracic anatomy of two cadavers (1 male and 1 female). The pulmonary artery (PA) dimensions, distance to major structures and nerve supply were determined by anatomical inspection.
HISTOLOGICAL
The pulmonary arteries were excised en bloc and stained as previously described20, using rabbit anti-tyrosine hydroxylase antibody (1:300, AB152; Merck Millipore, Burlington, MA, USA) and neurofilament (1:500, Ab24574; Abcam, Cambridge, United Kingdom) primary antibodies with species-specific secondary antibodies. Nerve location, area and distance from the vessel lumen were measured. Consecutive slides were projected onto a Cartesian map to form a two-dimensional (2D) representation of the nerve distribution. The data presented represent the distribution of individual nerves in the patients examined.
COMPUTATIONAL MODELLING: FINITE ELEMENT ANALYSIS
A simplified simulation of induced heat in tissue following ultrasound energy delivery was performed using 2D Cartesian numerical models (MATLAB R2009; MathWorks, Natick, MA, USA).
A constant diffusion coefficient per tissue type was assumed through time and piecewise constant through space reducing the equation to:
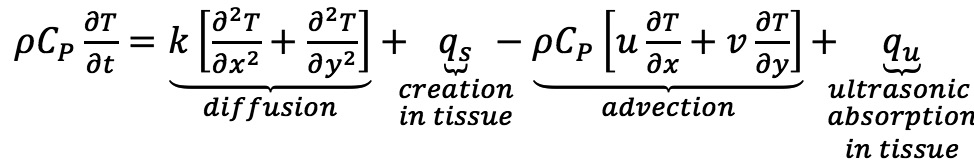 24
24
T-temperature profile; ρ-density; CP-capacity; qs-metabolic heat generation; qu-heat absorption (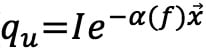 )25; I-intensity; α-attenuation coefficient; f-frequency;
)25; I-intensity; α-attenuation coefficient; f-frequency; 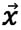 -position vector along the ultrasonic beam.
-position vector along the ultrasonic beam.
The model used a Crank-Nicolson method and comprised five concentric layers describing the region of interest geometry: lumen 25 mm, intima 0.02 mm, media 0.94 mm, adventitia 0.04 mm and perivascular tissue, with each layer defined by density, heat capacity, conductivity, metabolic heat generation and volumetric blood perfusion25. Transducer frequency was 11 MHz with an artery attenuation level of 15 dB/cm and coagulation necrosis threshold of 47°C. Transient effect was assumed to be negligible as tissue achieved steady state of >47°C in <3 seconds.
ULTRASOUND ENERGY DELIVERY EX VIVO
An ultrasound catheter (TIVUS; SoniVie, Rosh Ha’Ayin, Israel) was clamped submerged in a water bath and positioned using an XZY manipulator 8 mm from an ex vivo bovine liver sample. The three-directional ultrasound applicator of the catheter is a 2 mm diameter circumcircle of the equilateral triangle with a transducer of 6×1 mm on each side powered at 50 W/cm2 with an operating frequency of 11 MHz.
ANIMAL STUDIES
Fifteen male swine were used for examination of the acute histological changes induced by PDN, seven to determine the haemodynamic efficacy of PDN and ten to determine the effects of PDN at 14, 28 and 95 days. Anaesthesia was induced by intramuscular injection of ketamine (10 mg/kg), xylasin (2 mg/kg) and intravenous diazepam (1 mg/kg) and maintained with isofluorane 1.5-2.5% in 100% O2 via endotracheal tube. Interventional procedures were performed with radiographic guidance (H-5000 Integris Cath Lab; Philips, Amsterdam, the Netherlands). Studies were conducted under ethical approval 950/14/ANIM from the Sheba Medical Center, Ramat Gan, Israel.
HAEMODYNAMIC MEASUREMENTS
Systemic and pulmonary pressures were measured using a 5.2 Fr pigtail catheter (Cordis, Cardinal Health, Milpitas, CA, USA) via a 6 Fr sheath in the femoral artery and a 7 Fr Swan-Ganz catheter (SG) (Edwards Lifesciences, Irvine, CA, USA) via an 8 Fr sheath in the right internal jugular vein (Cordis).
PULMONARY DENERVATION
PDN was performed using a multidirectional ultrasound catheter (TIVUS) and console (Figure 1). Animals were randomised to PDN or a sham procedure (dummy catheter) undertaken via the internal jugular vein. Weight, HR and blood results are provided in Supplementary Table 1. The SG was advanced to the pulmonary artery and a 0.025”×260 cm wire (GuideRight™; St. Jude Medical, St. Paul, MN, USA) passed through the distal lumen; the SG was exchanged for a guiding catheter (RDC1; Cordis). The bifurcation of the main pulmonary artery and the ostium of the basal segmental branches were used as proximal and distal anatomic markers20. On a 0.014” wire, the TIVUS catheter was positioned and activated with the distancing mechanism open. Continuous monitoring of output and temperature with automated cut-out ensured that the ultrasound probe did not contact the vessel wall during activation. The number of activations was dependent on anatomy, limited to ten per vessel.
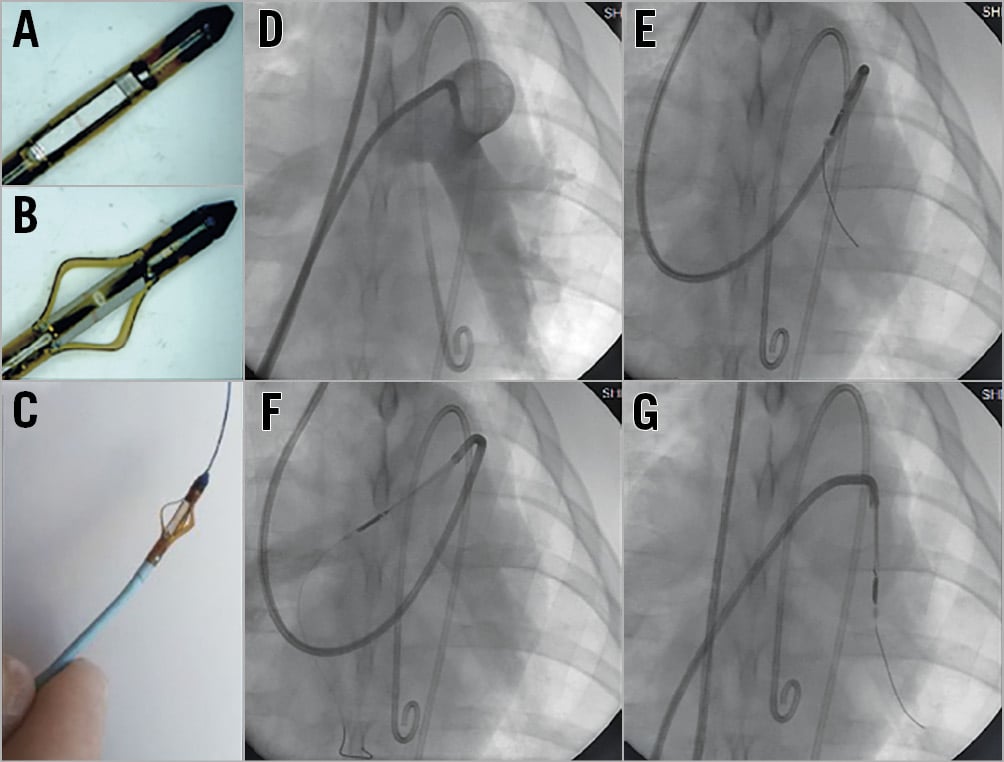
Figure 1. TIVUS catheter. A) - C) Catheter with distancing device closed and open and on a 0.014” wire. D) Pulmonary angiogram. E) - G) Fluoroscopic images of the TIVUS catheter in right, main and left pulmonary arteries.
THROMBOXANE-INDUCED PULMONARY HYPERTENSION
Acute PH was induced infusing thromboxane A2 agonist (TxA2, D0400; Sigma-Aldrich, St. Louis, MO, USA)20,26 via a 6 Fr sheath in the right internal jugular vein. As previously described20, the dose of infused TxA2 was increased at five-minute intervals, providing a dose-dependent increase in pulmonary artery pressure. Following the withdrawal of TxA2, pressure returned to baseline. PDN was then performed and TxA2 infused, identically to pre-treatment.
HISTOLOGICAL SCORING
Vessel scoring quantified endothelial loss, medial injury, inflammation, soft tissue injury and necrosis by light microscopy using H&E stained sections on a grading system (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe)27,28.
Nerve scoring quantified perineural inflammation or fibrosis and endoneural injury including vacuolisation, digestion chambers, pyknotic nuclei, and necrosis using a grading system (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe)27,28.
QUANTIFICATION OF SYMPATHETIC STAINING
Images of tyrosine hydroxylase (TH) stained sections were imported into ImageJ macOS X (NIH), threshold set, converted to greyscale and inverted for automated quantification of positive staining (CellProfiler 3.1.5macOS; Broad Institute)29.
STATISTICAL ANALYSIS
Data are expressed as mean and/or SD. Differences were assessed by Student’s t-test or Mann-Whitney test as appropriate in Prism 7.0d (GraphPad Software, San Diego, CA, USA).
Results
INNERVATION OF THE HUMAN PULMONARY ARTERIES
To determine the innervation of the pulmonary arteries and the proximity of local structures, anatomical and histological studies were undertaken in human cadavers. Macroscopic inspection demonstrated that the pulmonary plexus originated from the vagus nerve and the spinal ganglions. Parasympathetic nerves from the vagus were located caudal and anterior to the main pulmonary artery and sympathetic nerves from the spinal ganglions supplied the posterior aspect. Sympathetic and parasympathetic nerves merge to form a plexus, which was larger around the main pulmonary artery, splitting and reducing in size after the bifurcation of the main pulmonary artery. Other than intended targets, only the left recurrent laryngeal nerve was present within the 10 mm treatment area.
To define the distribution around the proximal pulmonary arteries, histological analysis evaluated nerve number and area. The number and area of nerves at the main pulmonary artery was greater than the left and right. Nerves were present at a depth of 1-12 mm with 44.7% and 46.2% surrounding the left and right pulmonary arteries at a depth >4 mm, respectively (Figure 2, Supplementary Table 2). Seventy-one percent (71%) of nerves stained positive for TH, indicating a predominantly sympathetic innervation (Supplementary Table 2).
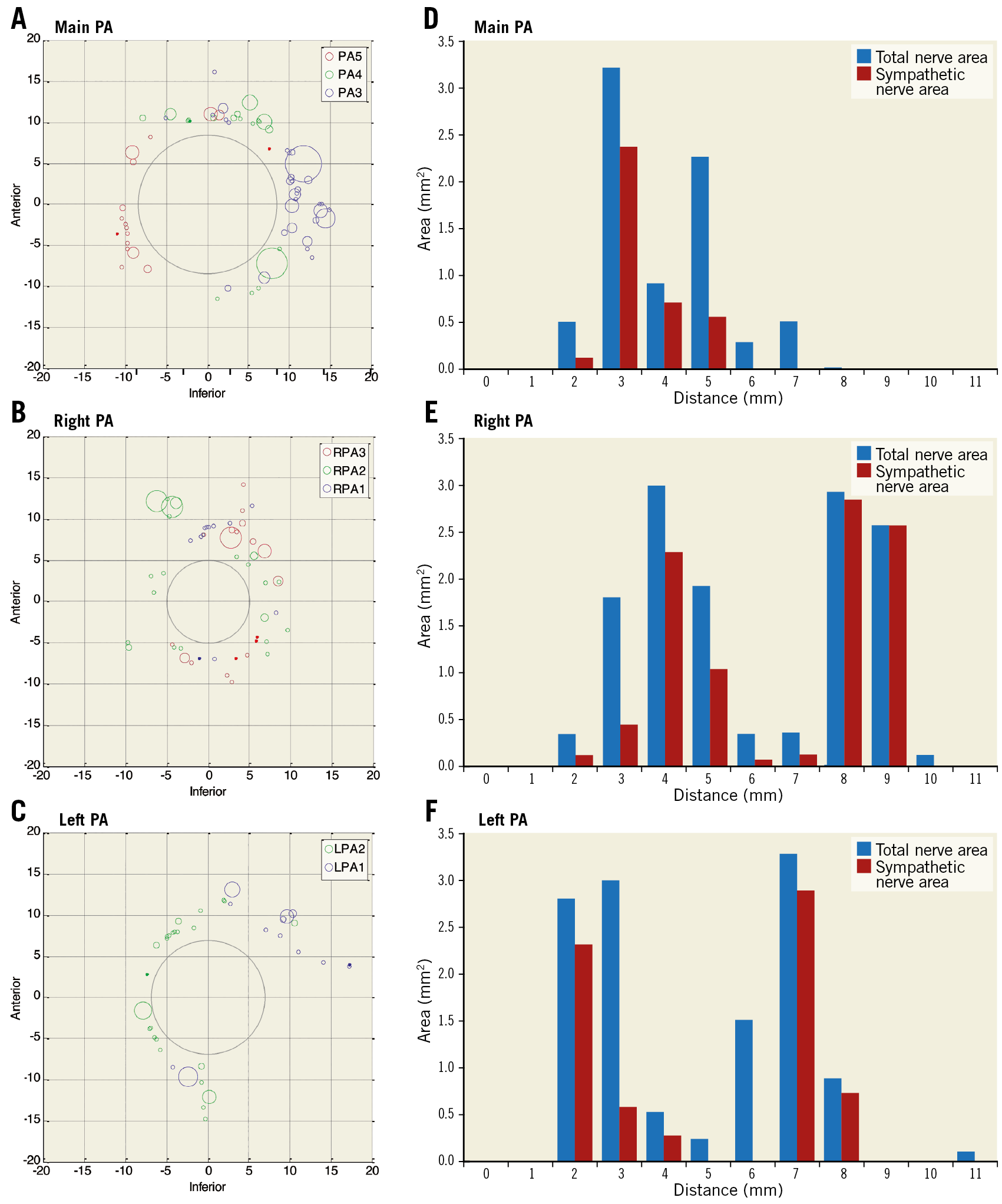
Figure 2. Human pulmonary artery nerve distribution. A) - C) Cartesian representation of nerve distribution at the main, right and left pulmonary arteries in two patients at three sequential 3 mm sections (blue, green, red). Circles represent nerve area (mm). D) - F) Cumulative area and depth of sympathetic and total nerves from the luminal aspect of the pulmonary artery.
COMPUTATIONAL SIMULATION OF ULTRASOUND ENERGY DELIVERY
Computational simulation of ultrasound energy delivery was used to model the effect on the pulmonary artery wall. Figure 3A shows computational modelling, suggesting that an ultrasound transducer located within the vessel lumen delivers energy to a depth of 0.5-9.5 mm. To demonstrate the effect on tissue, ultrasound energy was delivered to ex vivo bovine liver sections suspended in a static water bath at a distance of 8 mm. Consistent with computational models, the thermal effect matched the predicted distribution of temperatures >47°C. The cooling effect of the water interface prevented an effect at the water-tissue interface (Figure 3B).
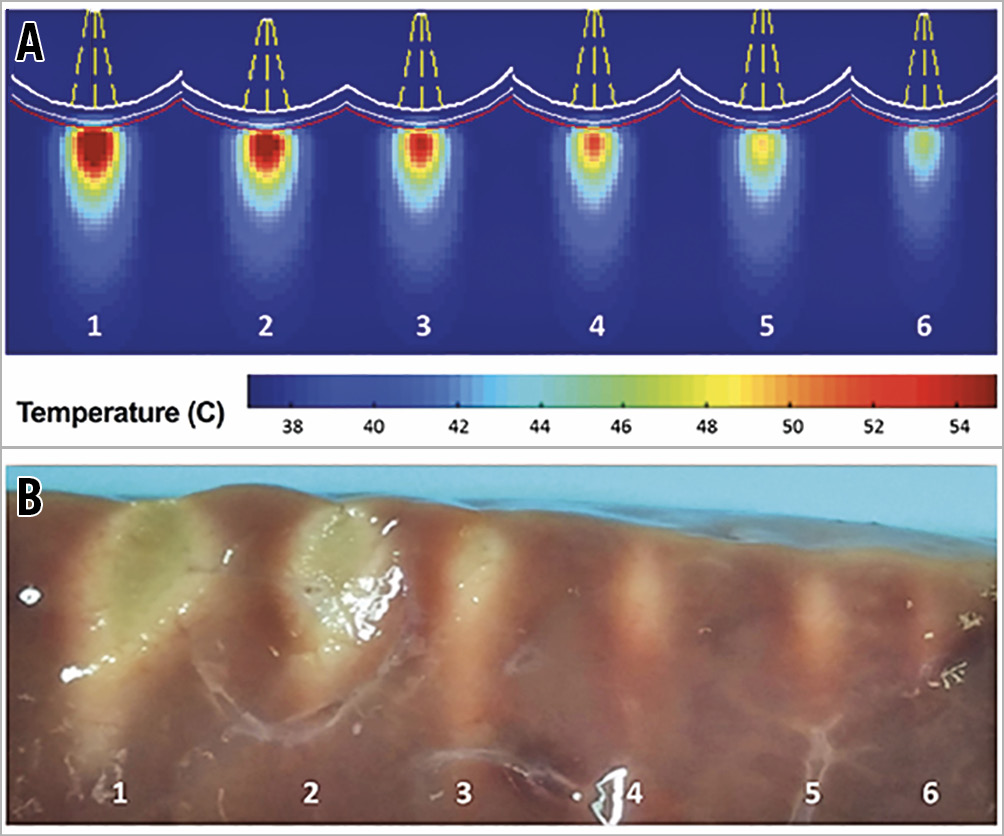
Figure 3. The thermal effect of ultrasound energy delivery in tissue. A) Computational simulation of the thermal effect to tissue following exposure to varied ultrasonic intensities. B) Thermal effect in bovine liver following ultrasonic energy delivery (white-thermal effect).
ULTRASOUND PULMONARY ARTERY DENERVATION INDUCES ACUTE HISTOLOGICAL CHANGES
To demonstrate the thermal effect on nerves, PDN was performed in domestic swine. Histological examination showed coagulation of collagenous connective and adipose tissue, and coagulation, vacuolation and pyknosis nerves (Figure 4A-Figure 4C) indicating an acute localised thermal effect.
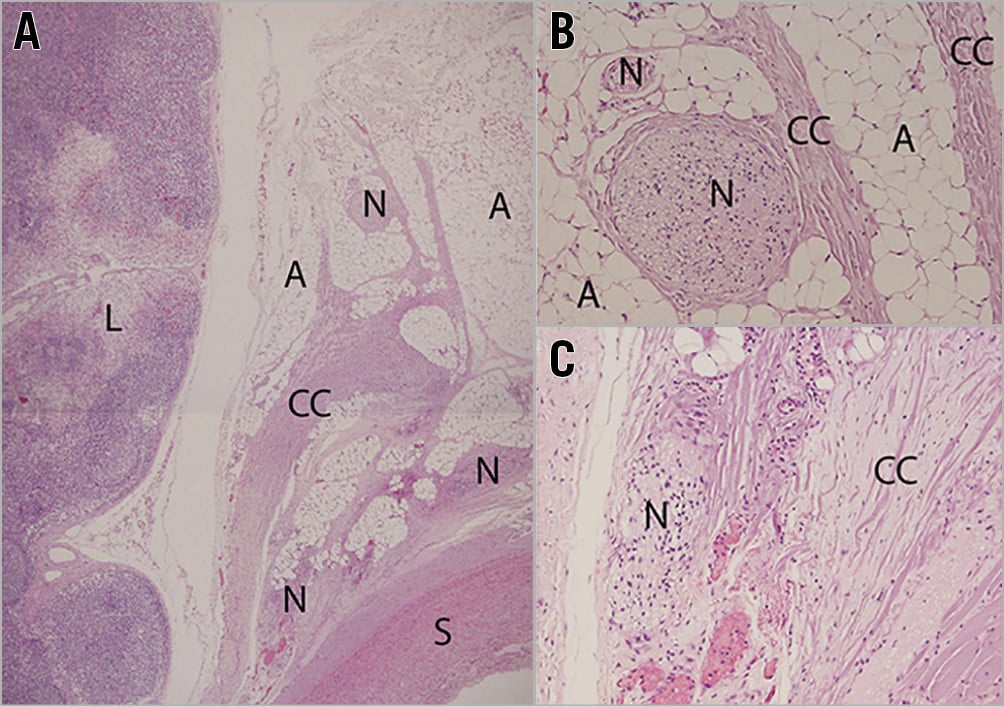
Figure 4. The acute effect of ultrasound PDN. A) - C) H&E stained sections demonstrate thermal effect indicated by coagulation of collagenous tissue and nuclei pyknosis of cells including nerves. The intima and media are spared as are local lymph nodes (A: adipose, CC: coagulated collagen, L: lymph, N: nerve and S: smooth muscle cells).
ULTRASOUND PULMONARY ARTERY DENERVATION REDUCES PULMONARY ARTERIAL PRESSURE
Administration of TxA2 resulted in the development of acute pulmonary hypertension in domestic swine, as demonstrated by a mean pulmonary artery pressure (mPAP) greater than 40 mmHg in both the control and PDN groups (Figure 5). PDN was performed following the first TxA2 challenge. In the control group, the achieved mPAP response was consistent between the first and second TxA2 infusions. PDN reduced mPAP during the second TxA2 challenge when compared to the sham control group (Figure 5). No significant effect was observed on systemic blood pressure or heart rate.
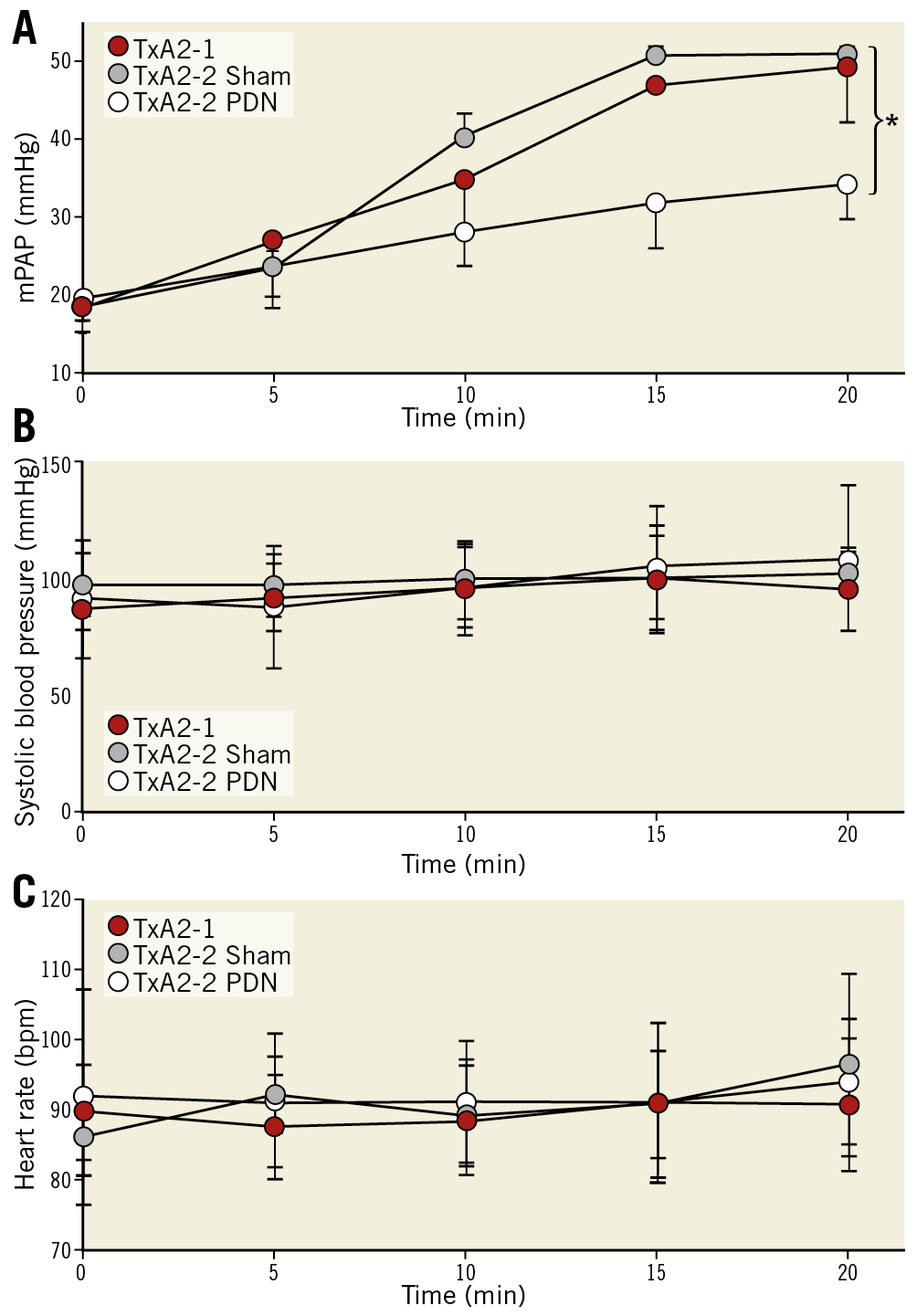
Figure 5. Ultrasound PDN reduces TxA2 induced PH. Mean pulmonary artery pressure (A), systolic blood pressure (B) and heart rate (C) in sham and PDN treated animals (mean±SD, *p<0.05, Student’s t-test).
SAFETY
No pulmonary artery dissection, perforation or rupture was identified, and no charring or thrombus formation was present during the studies. The animals were kept under surveillance for 14, 28 and 95 days post PDN. No abnormalities were identified in routine blood investigations, activity or weight gain, and no cardiorespiratory distress was identified (Supplementary Table 1).
DURATION OF HISTOLOGICAL CHANGES FOLLOWING PDN
To determine the long-term effect of ultrasound PDN on the vessel wall, histological examination was undertaken in treated animals at 14, 28 and 95 days. Macroscopic assessment showed no damage to the heart, no pulmonary artery thrombus or charring, and no aneurysm, dissection, perforation or stenosis of the pulmonary arteries or the adjacent aorta. Focal areas of neointima 150-250 μm in depth were present (Supplementary Figure 1) with no evidence of lumen reduction (defined as a cross-sectional reduction >5%). Within the adventitia, localised areas of fibrosis affecting connective, adipose and nerve tissue were observed at days 14, 28 and 95 days post PDN at a depth of 0.5-9.5 mm (Figure 6, Figure 7, Supplementary Table 2). The presence of giant cells, eosinophils and neutrophils at day 14 demonstrated an early inflammatory response, which was no longer present at 95 days. Large orbicular lipid droplets indicated a thermal effect in adipose tissue, while the fibrosis and thickening of the epineurium persisted to 95 days post PDN, indicating long-term alteration of the nerve structure (Figure 6, Figure 7, Supplementary Table 2). TH staining was reduced 95 days post PDN, demonstrating a long-term effect on sympathetic nerves (Figure 8, 0.190.09 vs 0.050.03, p<0.0001). Histological examination of adjacent structures including the lungs and aorta demonstrated no thermal effect.
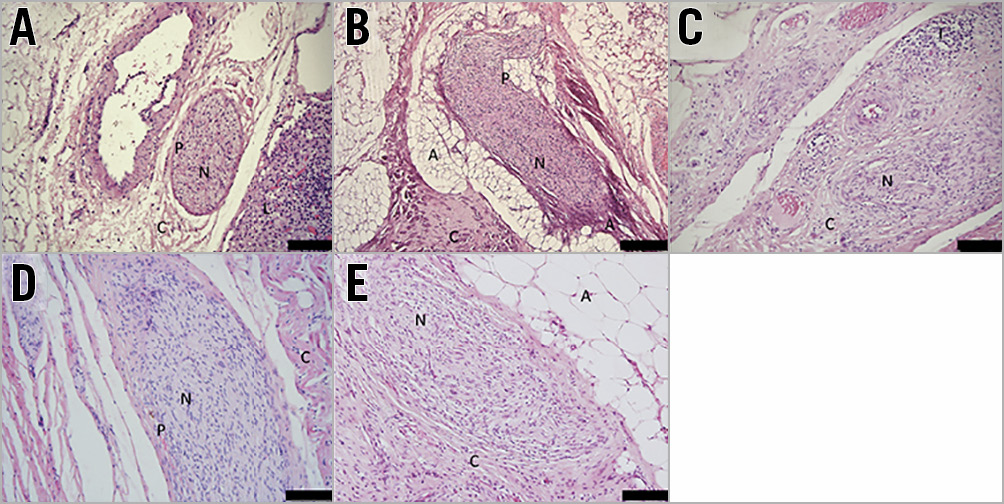
Figure 6. Time course of thermal injury following PDN. A) Control. B) Acute thermal effect with coagulation of nerve and collagenous connective tissue. C) - E) Day 14, day 28 and day 95. Ultrasound PDN is associated with long-term endoneural changes of nerves in the pulmonary artery adventitia demonstrated by the increased cellularity, perineuronal inflammation and fibrosis (H&E stained sections; A: adipose tissue; C: collagenous connective tissue, N: nerve; P: epineurium).
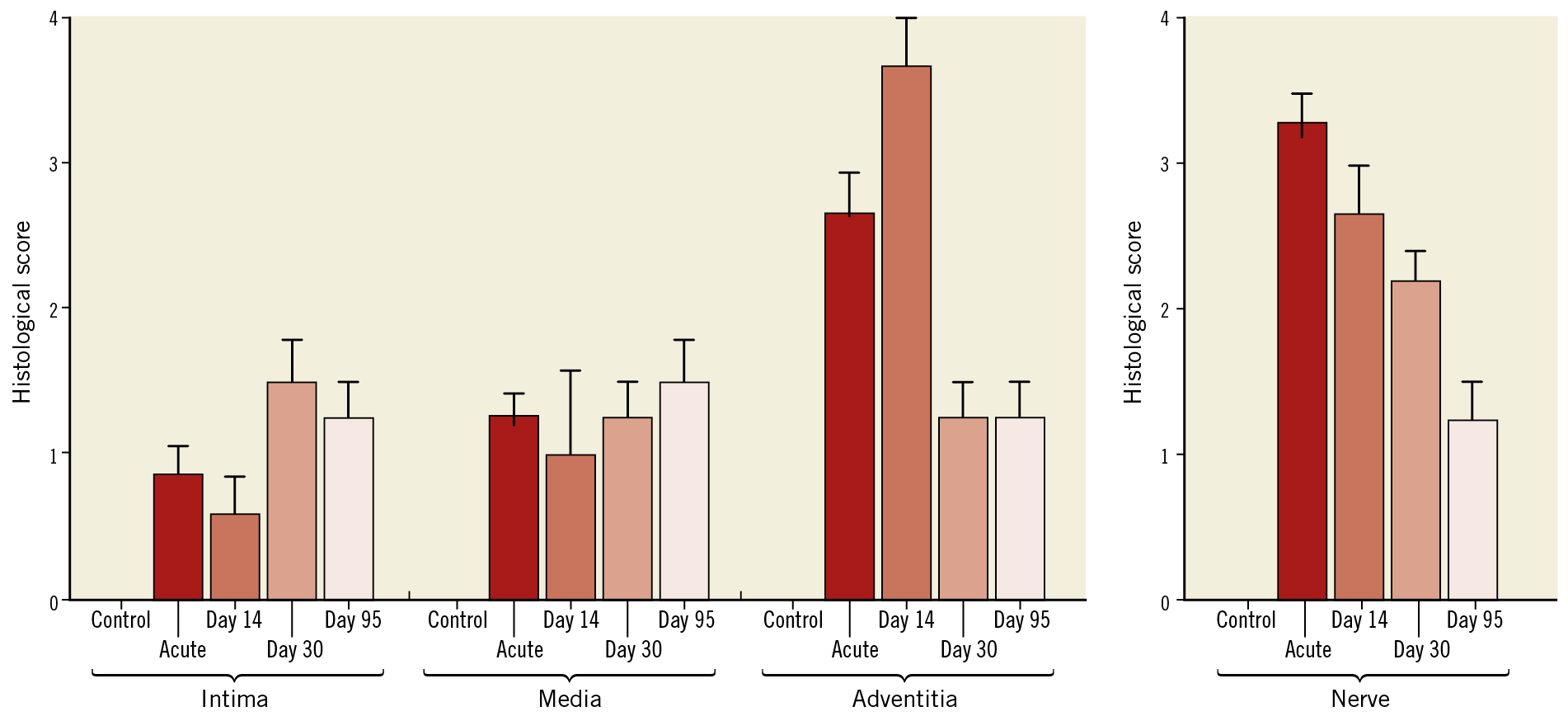
Figure 7. Quantification of vessel and nerve injury following ultrasound PDN. Vessel scoring quantified endothelial loss, medial injury, inflammation, soft tissue injury and necrosis (0-none to 4-severe). Nerve scoring quantified perineural inflammation or fibrosis and endoneural injury including vacuolisation, digestion chambers, pyknotic nuclei, and necrosis (0-none to 4-severe).
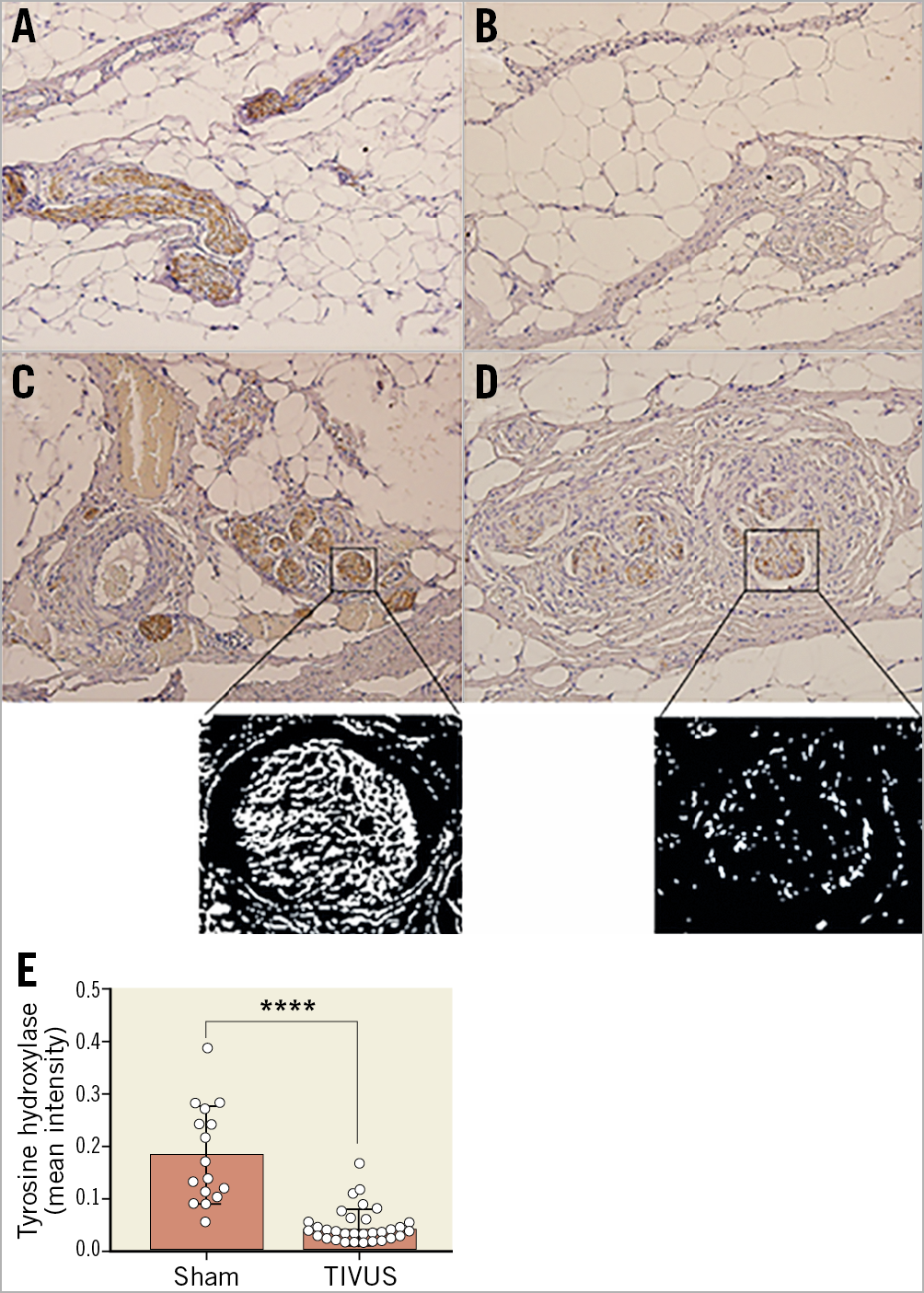
Figure 8. Ultrasound PDN results in reduced tyrosine hydroxylase staining at 95 days post procedure. Tyrosine hydroxylase (brown) stained pulmonary artery nerve sections at day 95. A) Control. B) - D) PDN. Magnified sections of panels C and D represent threshold-adjusted, colour-inverted images used for quantification of TH staining. E) Pulmonary artery nerve TH staining in PDN and sham treated animals (meanSEM, ****p<0.0001, Mann-Whitney test).
Discussion
The lungs and pulmonary vasculature release and metabolise >40% of circulating norepinephrine30. Sympathetic activity is increased in patients with PAH5 and identifies patients with an adverse prognosis6. These findings suggest that sympathetic modulation may be a potential target for treatment of PAH.
Neurohormonal modulation is an established strategy for the treatment of heart failure, reducing morbidity and mortality8,9,10,11,12,13. An 18-patient trial of bisoprolol in patients with idiopathic PAH reduced heart rate and increased stroke volume; however, it reduced cardiac output and the six-minute walk test17. As such, targeting nerves that supply the pulmonary vasculature without an adverse effect on cardiac function is an attractive concept. Percutaneous technologies developed for renal denervation reduce systemic blood pressure in patients with hypertension both off31,32 and on33 antihypertensive medication in randomised, sham-controlled studies and provide the opportunity to investigate the effect of denervation of the pulmonary artery for the treatment of pulmonary hypertension.
In humans, the pulmonary arteries are supplied by a predominantly sympathetic innervation (71%) that is circumferential in distribution with 40% at the left and right pulmonary arteries at a depth >4 mm. Computational modelling and ex vivo studies demonstrated delivery of ultrasonic energy in tissue to a depth of 9.5 mm, sparing the tissue-water interface that represents the vessel intima and media. In vivo histological alterations of nerves in the pulmonary artery adventitia following ultrasonic PDN were consistent with previous reports of radio-frequency (RF) renal28 and pulmonary20 denervation with altered structure, demyelination and reduced TH immunostaining21. RF energy results in local energy delivery altering the histological structure of the intima, media and adventitia to a depth of ~3 mm20,21. The present study demonstrates histological changes to nerves at a depth of 9.5 mm with limited effects on the pulmonary artery intima and media. This may be of benefit in patients with PAH in whom the media is thickened and the distance from the lumen to the adventitia is increased34.
Incomplete ablation has been proposed as a potential mechanism of failure in renal denervation. The TIVUS system provides a semi-circumferential energy delivery with three externally directed beams. In vivo studies have demonstrated that a three-directional ultrasound probe in a 2.5 cm pulmonary artery results in structural alteration of ~80% of the vessel circumference (Supplementary Figure 2). We used human anatomical data to develop a structured procedure to target the majority of the pulmonary vascular innervation through multiple activations, similar to techniques used to provide a “complete” renal ablation.
Consistent with prior reports of RF-based denervation in balloon-distension19, TxA220 and monocrotaline21 models of PH, ultrasound PDN improved pulmonary haemodynamics in an acute vasoconstrictive model of PH as demonstrated by a reduction in mPAP. Histological evidence of denervation following ultrasound energy delivery was present 95 days post PDN with altered nerve structure and staining to a depth of 9.5 mm. These data suggest that ultrasound energy delivery may target a greater proportion of the nerves innervating the pulmonary vasculature than RF energy. No arrhythmias or significant changes in heart rate or systemic pressure were identified during follow-up. The left and right ventricles are interdependent, and at increased right-sided pressure the interventricular septum displaces leftward, altering the shape, filling and function of the left ventricle. This effect may mask physiological effects of PDN on systemic pressure in models with increased right-sided pressures.
The first clinical study of PDN in patients with idiopathic PAH provided early evidence of clinical benefit22. Subsequent reports have broadened the patient groups treated, providing further promising results35. These studies are single-centre and limited in patient number. Due to study design, there are to date few data to inform understanding of the longevity of the haemodynamic improvements identified or provide evidence of a therapeutic effect in patients treated with PAH-specific therapies.
Strengths and limitations
PAH in humans is driven by pulmonary vascular remodelling and vasoconstriction. Acute haemodynamic changes in the TxA2 model suggest that ultrasound PDN modulates pulmonary vasoconstriction, but provide no data relating to remodelling. The demonstration of altered nerve structure and staining in vivo and the description of the anatomical distribution of nerves around the pulmonary vasculature in humans demonstrate that nerves supplying the pulmonary vasculature are accessible via a percutaneous ultrasound-based procedure. As such, these data provided a basis for clinical studies of ultrasound pulmonary artery denervation in PAH, a condition for which all disease-specific therapies target vasoconstriction1.
Conclusions
The present study describes the distribution of innervation of the proximal pulmonary vasculature and demonstrates that ultrasonic energy delivered by a percutaneous catheter-based system results in sustained alteration in the structure and histochemical staining of sympathetic nerves, sparing the vessel intima and media. Additionally, ultrasound PDN reduced mPAP in an acute porcine model of PH.
|
Impact on daily practice Patients with pulmonary arterial hypertension continue to experience significant morbidity and mortality. Therapeutic intravascular ultrasound pulmonary artery denervation alters nerve structure and reduces pulmonary artery pressure in animal models of pulmonary hypertension. Further clinical studies are required to determine efficacy in patients with pulmonary arterial hypertension. |
Acknowledgements
A.R. Tzafriri thanks Fernando Garcia-Polite (CBSET) for technical support.
Funding
Studies were funded by SoniVie, Israel. A. Rothman is supported by a Wellcome Trust grant (206632/Z/17/Z).
Conflict of interest statement
A. Rothman, M. Jonas, M. Leon, O. Ben-Yehuda and L. Rubin have consulted for SoniVie. D. Shav is employed by SoniVie. A.R. Tzafriri, D. Castel, and H. Traxler report grants from SoniVie.

