
Pulmonary hypertension (PH) describes an abnormal pressure elevation in the lesser circulation that accompanies ageing, as well as cardiovascular and pulmonary disease, and many other systemic diseases. Pulmonary arterial hypertension (PAH) is a subset of PH that results from pulmonary vascular disease, an obliterative disorder that affects the precapillary <200 µm diameter arteriolar vessels, the capillary bed, and the small post-capillary venules. The mechanisms responsible for vasculopathy in PAH are poorly understood but probably involve vasoconstriction, altered vascular growth and proliferation, and potentially a central nervous system regulatory axis1,2,3. In support of this concept, experimental PH by distention of the pulmonary artery (PA) is produced by excitation of stretch receptors in or near the bifurcation of the main PA, and the efferent limb of this reflex is predominantly mediated via the adrenergic nervous system4. However, while in primary systemic hypertension of younger age high sympathetic nerve activity responds well to β-blocker treatment, clinical studies using β-blockers in PAH are limited and non-randomised5,6, only a few suggesting safety and possible benefits7.
All currently approved PAH therapies target one or more dysfunctional pathways that normally control pulmonary vasomotor tone, thus providing a relatively small afterload decrease for the right ventricle (RV). While a significant impact on survival in PAH has been achieved by this concept, PAH is still a devastating condition with a high annual attrition rate of around 8%. New treatment concepts are needed, particularly outside of endothelin receptors, cAMP and cGMP pathways in order to enhance possible additive effects. PAs are highly innervated, metabolising >40% of circulating catecholamines8. Therefore, PA denervation (PADN) has been gaining ground in recent years, with extensive animal experimentation9 (Table 1), and first clinical studies (Table 2), and an event-driven trial under way (PADN-PAH, NCT02284737).
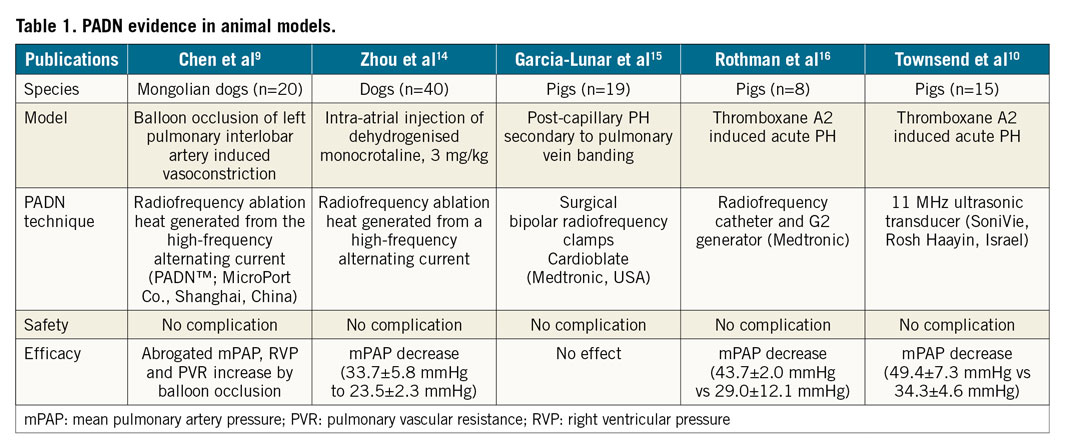
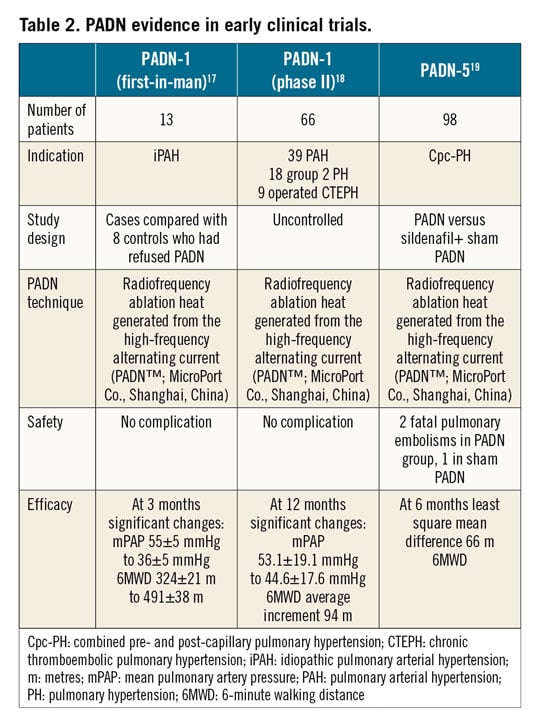
In this issue of EuroIntervention, Rothman et al10 are providing important details of pulmonary vascular innervation in human cadaveric and histological specimens.
The authors show that pulmonary (nerve) plexus is predominantly sympathetic (~70%) and is innervated by fibres from the spinal ganglions (sympathetic) and vagus nerve (parasympathetic). Sympathetic nerves are concentrated on the PA bifurcation, with >40% of nerves at a depth of >4 mm, tracking the arterial course distally (Figure 1).
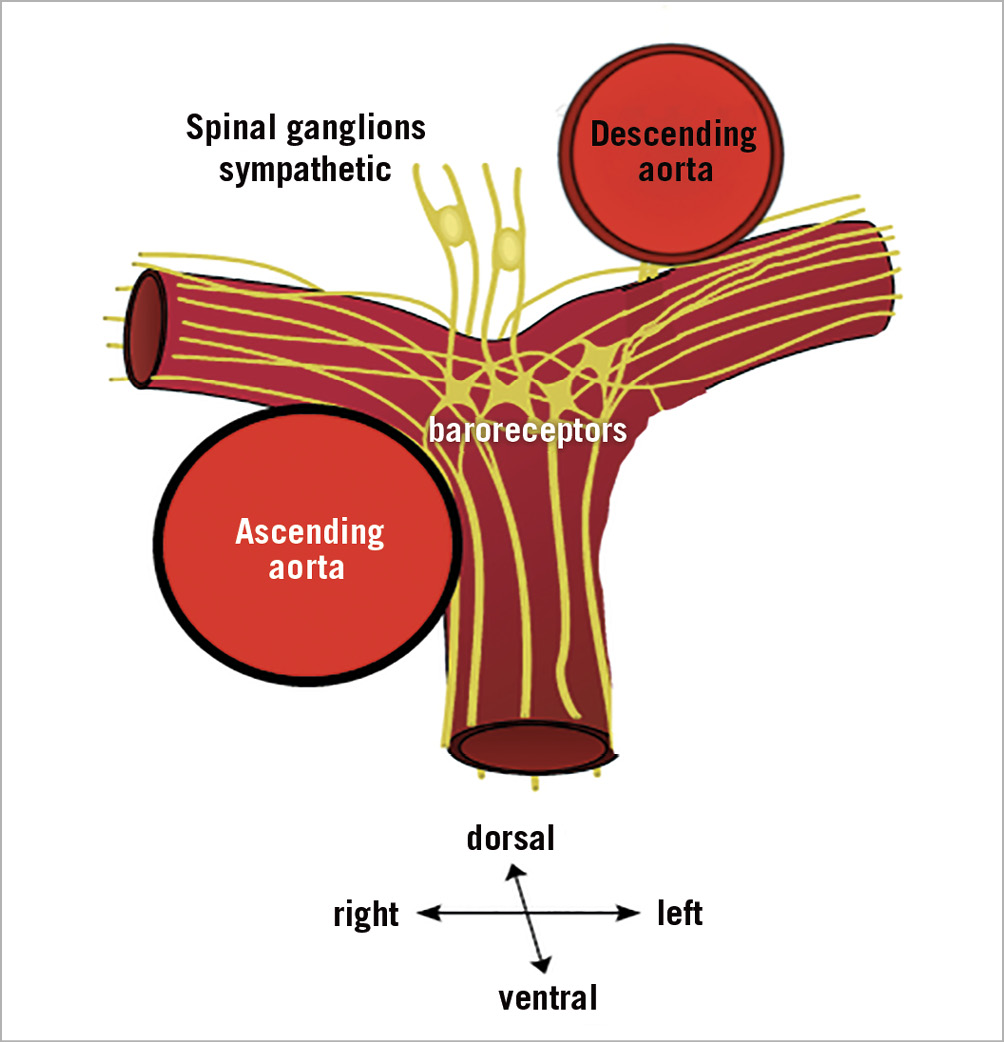
Figure 1. Diagram of PA nerve density in different segments of the human PA. Nerves are between 3 and 8 mm abluminal to the endothelial surface, with around 3 mm depth from the inner lumen in the PA trunk. High nerve density is observed in the ventral aspect of the central part of the left PA, suggesting central PAs as the main treatment targets.
Using computational modelling ex vivo and a swine model, the authors tested the feasibility of using an intravascular ultrasound-based energy delivery system for PADN. They found it to be safe; structural changes in PA nerves persisted up to 95 days post procedure. This work lends support to earlier studies in dogs where sympathetic nerve conduction velocity, axon diameter, and myelin thickness were found to be markedly increased and returned to normal after PADN. Taken together, proof of concept and safety of radiofrequency ablation and ultrasound energy-based ablation have been confirmed in animal models; Rothman et al are the first to provide anatomical information on human PA sympathetic innervation. Clinical data on PADN are sparse, with the first European safety trial TROPHY (NCT02516722) soon to be published. The next steps will have to be guided by the renal denervation trials. After the sham-controlled SYMPLICITY HTN-3 trial dampened the euphoria following early renal denervation trials, the recently published results of the sham-controlled SPYRAL HTN11 and RADIANCE-HTN12 trials have provided new biological proof of principle for the blood pressure-lowering efficacy of renal denervation. The same rigorous standards as in these trials will have to be adopted by the PADN trials; however, critical differences between the systemic and pulmonary circulation have to be taken into account. First, PAH is a life-threatening condition where discontinuation of medications may lead to rapid deterioration and must be considered unethical. Second, sham procedures in severely ill patients are associated with unacceptable procedural risk, which will lead to enrolment of less severe patients. Third, in radiofrequency renal denervation studies, combination treatment of the main artery plus more distal branches produced the greatest decrease in renal noradrenaline levels and axon density. However, this concept has not been tested in PADN, where catheter ablation has been performed within a few mm distal to the main PA bifurcation, and may be relevant because downstream pulmonary vascular resistance accounts for most of the total vascular resistance13. Still, in contrast to trials in systemic hypertension that have suffered from the shortfalls of office versus ambulatory blood pressure measurements, the standard in PAH trials has been and should remain invasive confirmation of treatment effects.
Taken together, after extensive animal experimentation and clinical safety trials, the time has come for a sham-controlled blinded randomised clinical trial to settle the uncertainty that many PH experts still share about the effectiveness of PADN in patients who are in need of treatment escalation.
Funding
Austrian Science Foundation F54 (to I.M. Lang).
Conflict of interest statement
I.M. Lang reports receiving speaker honoraria and grants to the institution from AOPOrphan Pharmaceuticals AG and Actelion-Janssen, speaker honoraria from MSD, speaker honoraria and travel expenses from Medtronic and Ferrer. In addition, she was an investigator in the SoniVie PADN clinical trial.

