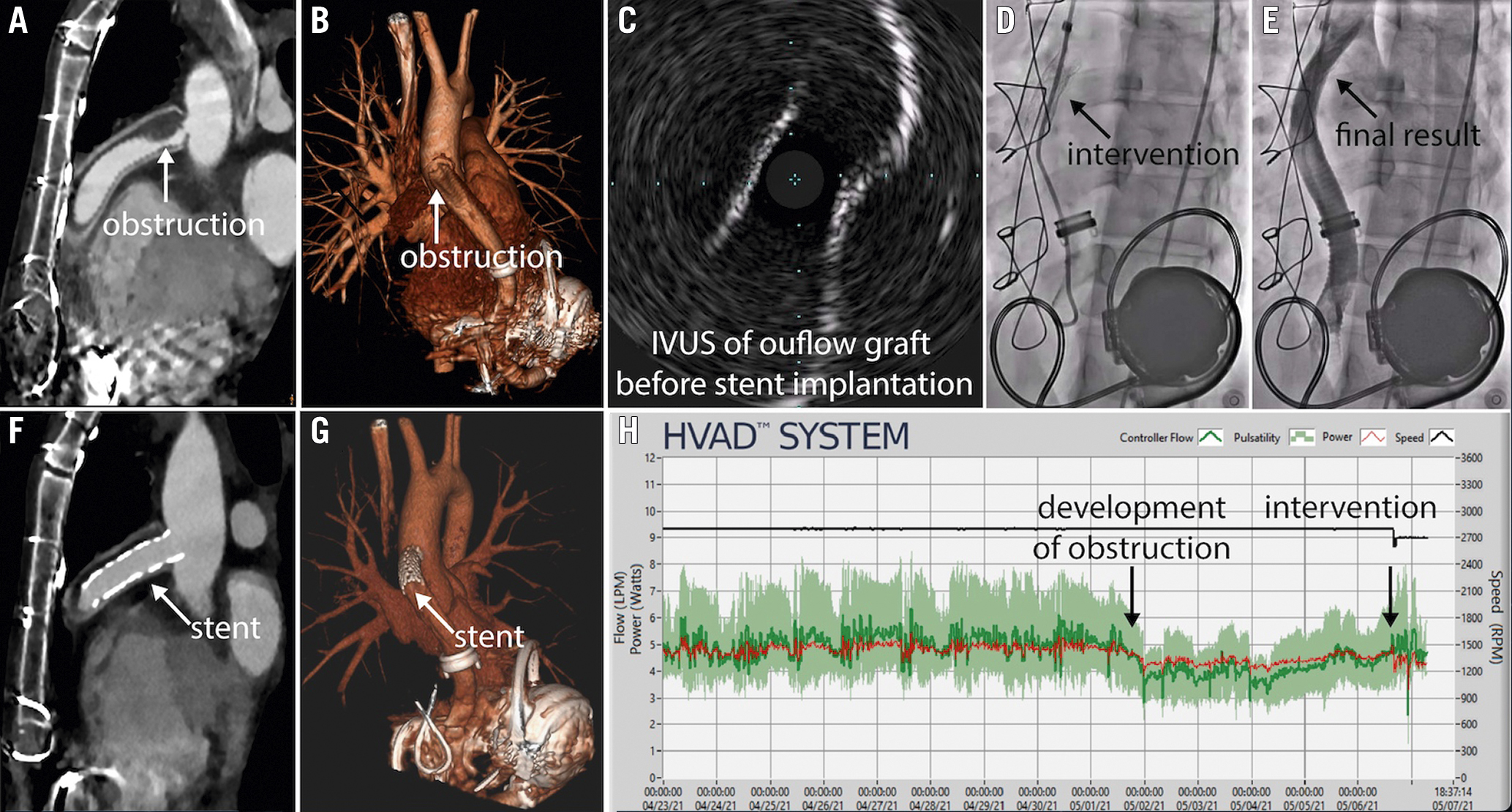
Figure 1. Multimodality imaging and treatment of left ventricular assist device (LVAD) outflow graft obstruction. A 37-year-old man who received a HeartWare ventricular assist device (HVAD) due to a pheochromocytoma with stress-induced, non-reversible cardiomyopathy four years earlier was admitted due to general malaise and low-flow alarms. Computed tomography (CT) angiography demonstrated HVAD outflow graft obstruction (A: white arrow; B: white arrow), which was confirmed with intravascular ultrasound (IVUS; C). Peak-to-peak pressure gradient was 75 mmHg. The stenosis was treated with a balloon-expandable covered stent (D: black arrow; Advanta V12, 12 mm×41 mm), leading to an increase in LVAD flow to 3.5 L/min. The control angiography showed a good procedural result (E: black arrow), which was confirmed by CT (F: white arrow; G: white arrow). The HVAD log showed a sudden decrease in pump flow four days prior to the procedure and restored normal flow following the intervention (H).
Left ventricular assist devices (LVAD) improve survival in patients with advanced heart failure1. LVAD outflow graft obstruction (OGO) is a potentially fatal complication, with the estimated incidence of 0.03 events per patient-year2. Percutaneous OGO management offers a less invasive alternative to surgery3. We present our experience based on five patients with OGO, who underwent percutaneous treatment. Patient baseline characteristics and procedural details are summarised in Supplementary Table 1. Three patients had a HeartMate 3 (Abbott) and two patients had a HeartWare (Medtronic) ventricular assist device. The mean time from LVAD implantation or replacement to OGO was 2.4 years. Clinical presentation included general malaise with low-flow alarms, progressive dyspnoea, acute decompensated heart failure, cardiogenic shock and out-of-hospital cardiac arrest (OHCA). Review of LVAD data log files showed various patterns of flow changes, including progressive decrease followed by sudden significant decrease in one patient, sudden decrease in two patients, sudden drop in flow in a patient who presented with OHCA and an increase followed by a decrease of flow in one patient. Intraluminal stenosis was diagnosed using computed tomography angiography (CTA) in four patients, except for one, who presented with OHCA and was transferred directly to the catheterisation laboratory. Before the intervention, all patients were discussed by the Heart Team and provided informed consent for the off-label use of peripheral endovascular equipment. All procedures were performed via a femoral artery approach (8 Fr-14 Fr) with conscious sedation. The outflow graft was engaged with an angled-tip catheter (multipurpose or pigtail) in a left anterior oblique (LAO) 40° view. A 0.035 wire was advanced into the outflow graft, with care taken to avoid wire interaction with the pump impeller. In all cases, angiography confirmed significant stenosis of the outflow graft with peak-to-peak gradients from 40 to 80 mmHg. In four cases, we proceeded directly from angiography to stenting. In one case, when graft twisting was suspected, balloon “graftoplasty” was performed with a 10 mm non-compliant balloon prior to stenting to ensure the feasibility of stent deployment (Advance; Cook Medical)45. We used balloon-expandable covered stents with a diameter of 10-18 mm (Advanta V12 [Getinge], n=4; BeGraft [Bentley], n=4). After stent deployment, we repeated angiography to evaluate for migration of the obstruction. In two patients, intravascular ultrasound (IVUS) was used to differentiate between the external and internal obstruction and confirm the adequate lumen diameter5. In a patient with diffuse obstruction, a cerebral embolic protection device was used (TriGUARD 3; Keystone Heart), followed by implantation of three balloon-expandable covered stents. Post-dilation was performed with a non-compliant balloon (Advance). The femoral artery puncture site was closed with a percutaneous closure device (Perclose ProGlide; Abbott). Control CTA was performed to evaluate the effect of the intervention. Three patients were discharged from the hospital: two following stenting and one following surgical replacement. Images of CTA, IVUS and/or angiography showing OGO diagnosis and treatment are shown in Figure 1, Supplementary Figure 1-Supplementary Figure 4. Based on our experience, percutaneous OGO management is a feasible and less invasive alternative compared to surgical revision. Although we used balloon-expandable covered stents due to their availability, external obstructions may be treated with a self-expanding, uncovered stent, allowing the sheath size to be decreased and the procedure to be performed using a single long stent. The safety and efficacy of percutaneous OGO management should be evaluated in future clinical trials.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

