
Introduction
The “chimney” and “BASILICA” techniques1,2 were developed to offer safe prevention of coronary artery occlusion (CAO) in case of valve-in-valve transcatheter aortic valve implantation (VIV-TAVI) in degenerated surgical bioprostheses. However, sometimes these lead to unnecessary stenting or may simply not be performed because of unfavourable anatomy. Moreover, when they are not implemented, a reassuring coronary angiogram after valve implantation may hide the risk of delayed CAO, which could occur after the removal of protective wires from coronaries3.
This study aimed to evaluate the feasibility and usefulness of intravascular ultrasound (IVUS) analysis of the coronary ostia to predict and prevent CAO after VIV-TAVI.
Methods
This prospective observational analysis included 50 patients undergoing VIV-TAVI in degenerated surgical bioprostheses, at the Verona University Hospital, from July 2011 to December 2019. This series is a part of the Verona Valve Registry approved by the local Institutional Review Board.
Except for the first three cases (before 2015), the remaining 47 underwent a meticulous protocol for the risk assessment of CAO4, taking into consideration the presence of the risk factors shown in Figure 1.
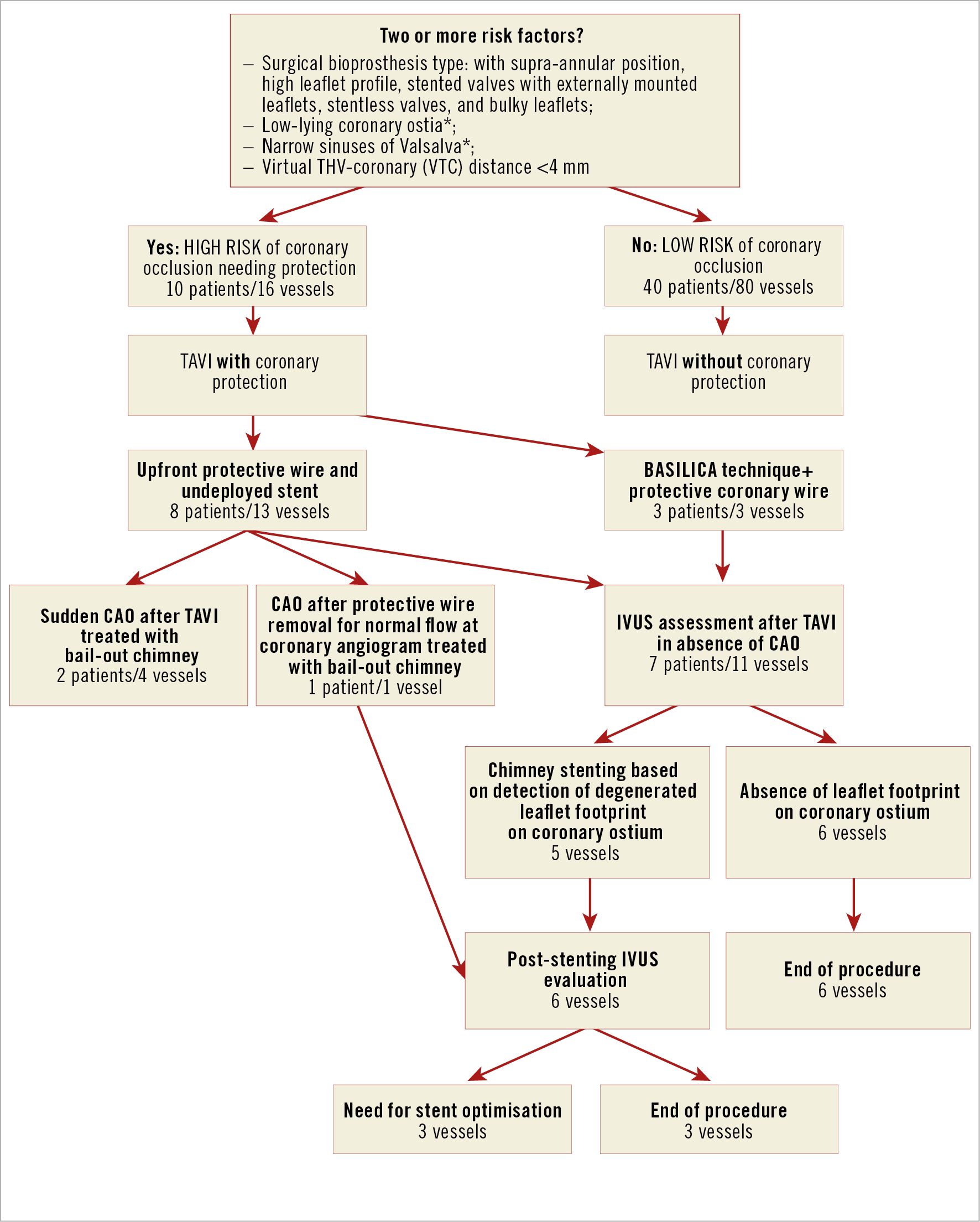
Figure 1. Management of VIV-TAVI in patients at high risk for CAO, according to the proposed IVUS-guided strategy. *suggested values are: <9 mm from the ostia to the sewing ring and <30 mm by width for Valsalva sinuses. CAO: coronary artery obstruction
Patients presenting ≥2 risk factors were considered at high risk of CAO and underwent coronary protection during the procedure, initially through upfront intracoronary guidewires with eventual bail-out “chimney/snorkelling technique” in case of sudden CAO2. From 2019, the bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) technique was introduced as an alternative in anatomically suitable cases.
The occurrence of CAO following removal of the protective wire from the left main led the team to consider a new strategy for the assessment of the true risk of CAO despite reassuring coronary angiograms after VIV-TAVI. Thus, IVUS assessment of coronary ostia at high risk of CAO following VIV-TAVI was systematically implemented. We hypothesised that IVUS could unmask possible CAO markers, not detectable by angiography (either selective or unselective) and potentially leading to acute or delayed CAO.
In brief, the IVUS strategy consists of the following steps:
– careful IVUS analysis taking into consideration the ostium, its relationship with the degenerated leaflet and any potential interaction with the transcatheter heart valve (THV) frame at the para-ostial space;
– in cases of partial obstruction, IVUS shows the degenerated leaflet footprint on the coronary ostia, considered a marker of an unsafe relationship between them (otherwise undetected by angiography). These findings, suggestive for high risk of acute or delayed complete obstruction, lead to stent implantation using the elective chimney/snorkel technique (Supplementary Figure 1, Moving image 1);
– conversely, the detection of fully patent ostia, without signs of leaflet footprint, supports the safe removal of the coronary wire, avoiding stent implantation (Supplementary Figure 2, Moving image 2);
– in case of stenting, a further IVUS evaluation is recommended to verify the patency and the correct stent apposition.
Results
Baseline characteristics of the 50 VIV-TAVI cases are available in Supplementary Table 1.
Ten patients (16 coronaries), out of the 47 evaluated according to our protocol, were considered at high risk for CAO and therefore underwent coronary protection (Supplementary Table 2). Acute CAO occurred in the first two patients protected with upfront intracoronary wires and undeployed stents, who were successfully treated by bail-out chimney. The third patient presented CAO, but only after the wire removal from the left main, following a misleading coronary angiogram showing a preserved flow after VIV-TAVI (Supplementary Figure 3).
Since then, in the absence of sudden CAO, IVUS was introduced as the primary strategy to assess the actual coronary patency before the wire removal, even after BASILICA (7 patients/11 coronaries) (Supplementary Table 3).
Five out of eleven vessels required stent implantation following IVUS evaluation, based on the presence of the degenerated leaflet footprint on coronary ostia leading to partial obstruction or on an unsatisfactory result of the cusp laceration following BASILICA. Conversely, IVUS allowed avoiding stenting in the remaining six vessels, due to the absence of markers suggestive of CAO.
Of note, none of these cases systematically approached with the IVUS strategy presented either haemodynamic impairment or signs of CAO during the procedure and the hospital stay.
The median hospital stay was 7 days (range 2-27). At the last available follow-up (range 6-108 months), neither myocardial infarction nor cardiac death was reported in any of the patients.
Discussion
To date, chimney/BASILICA techniques represent the only available strategies to prevent or manage CAO. Although studies reported low rates of stent complications after chimney2, the implantation of stents may render subsequent coronary re-catheterisations difficult. Even relying on wires left in place after VIV-TAVI to assess vessel patency may be misleading, with unexpected CAO and haemodynamic instability occurring after wire removal. Moreover, such complications may happen with a delayed onset, in particular in patients treated with self-expanding devices because of their tendency to expand further after implantation, therefore making a partial obstruction more likely to evolve into a complete CAO hours after the procedure.
Conversely, BASILICA, by avoiding stents, is less prone to complications, including difficulties in re-engaging coronary ostia. However, in some cases, BASILICA may not completely prevent CAO, due to the persistence of partial obstruction by a portion of the lacerated leaflet5, or because of the unfavourable anatomic relationship between the coronary ostium and the surgical valve commissures, making the laceration useless. Taking into consideration the limitations of these techniques, complementary strategies may be useful to ensure vessel patency better, to avoid unnecessary stenting, or to assess the effectiveness of BASILICA.
In the present report, IVUS showed signs of partial obstruction, not detected by the standard angiogram, in 5 out of 11 evaluated coronary arteries.
Of note, none of the six cases where the IVUS assessment excluded the need for stents suffered acute or delayed CAO, supporting the rationale of this technique.
The overall CAO rate in our series was higher compared to the VIVID registry (6% vs 3.5%)4. However, these data are closely related to the type of surgical bioprosthesis. Indeed, the VIVID registry reported a CAO rate of 30% among stentless prostheses, and up to 50% for the Freedom SOLO valves (Sorin Group Inc., Milan, Italy) which were the valves represented most in our series.
Based on these preliminary observational data, we propose the implementation of an “IVUS-assisted decision-making algorithm” in VIV-TAVI procedures (Figure 1), with the following goals:
– to guide the operators in assessing the need for possible stenting following VIV-TAVI;
– to support operators in avoiding unnecessary stenting;
– to expand the safety of BASILICA in difficult anatomies and presence of ambiguous angiographic imaging.
Limitations
Although the present report focuses mainly on the feasibility of this novel strategy, the definition of its efficacy and safety warrants further and larger studies, as well as the identification of a potential quantitative IVUS threshold to guide the operator’s decision better. Moreover, IVUS assessment after the valve implantation was performed with the wire into the coronary, that could keep the cusp of the surgical valve away from the coronary ostia.
Conclusion
IVUS assessment of coronary ostia after VIV-TAVI is proposed to detect potential markers of CAO. If validated in dedicated studies, this strategy could be implemented as a complementary tool in the algorithm for CAO prevention in TAVI.
|
Impact on daily practice The present study describes an IVUS-guided technique for the assessment of the coronary patency after VIV-TAVI and to support the operator in deciding whether to stent or, conversely, to avoid unnecessary stenting. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Approach to the case reported in Supplementary Figure 1.
Moving image 2. Approach to the case reported in Supplementary Figure 2.

