
Abstract
Aims: The behaviour of side branches (SBs) covered by a bioresorbable vascular scaffold (BVS) is not well known. This study analysed the rate of side branch occlusion (SBO) immediately after BVS implantation, its clinical impact, predictors of SBO and the fate of such SBs at follow-up.
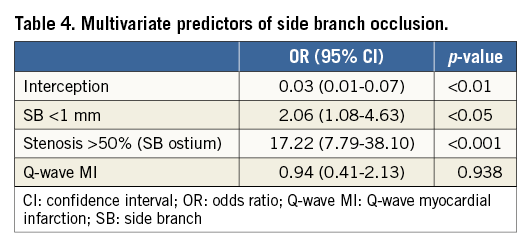
Methods and results: We assessed 140 patients with 346 jeopardised SBs divided into three groups: small (<1 mm, n=181), intermediate (1-2 mm, n=102) and large (>2 mm, n=63). SBO was defined as a TIMI flow 0 or 1. Computed tomography was scheduled at six months for patients with jailed SBs >1 mm. Immediate occlusion occurred in 31 (9%) SBs: 22 (12%) small, 8 (8%) intermediate and one (1.6%) large, while post-procedural SBO was 5.5%. In-hospital events included one thrombosis (0.7%) and eight non-Q-wave myocardial infarctions (6%). After 17±3 months, one patient died (0.7%) and six patients needed repeat revascularisation (4%). Re-evaluation showed no late SBO at 7±3 months. Predictors of SBO were: small SBs (OR 2.06, 95% CI: 1.08-4.63; p<0.05) and stenosis >50% at the origin (OR 17.22, 95% CI: 7.79-38.10; p<0.01).
Conclusions: The incidence of SBO and its clinical impact were low when SBs >1 mm were covered. These favourable results were maintained at midterm.
Abbreviations
BVS: bioresorbable vascular scaffold
CT: coronary computed tomography scan
DES: drug-eluting stent
IVUS: intravascular ultrasound
MACE: major adverse cardiac events
MI: myocardial infarction
OCT: optical coherence tomography
SB: side branch
SBO: side branch occlusion
SBs: side branches
Introduction
Fully bioresorbable vascular scaffolds (BVS) (Absorb; Abbott Vascular, Santa Clara, CA, USA) have been demonstrated to be effective in the treatment of non-bifurcated coronary lesions1-3. However, limited information is available regarding the fate of side branches (SBs) covered by a BVS. Moreover, with BVS it is not clear if struts covering the ostium of a side branch (SB) undergo a similar resorption process as compared with struts apposed to the vessel wall. The increased strut thickness of the BVS (157 µm) may be associated with a higher incidence of side branch occlusion (SBO) and, therefore, contribute to the development of periprocedural myocardial infarction, which is associated with unfavourable results at follow-up4-8.
The present study aimed to analyse the patency rate of SBs immediately after main vessel BVS implantation, to assess the clinical impact of SBO, to identify predictors of occlusion, and to study the fate of such SBs at midterm follow-up.
Methods
PATIENTS
Between January 2012 and April 2013, 149 patients with a total of 200 lesions involving at least one SB were treated by BVS implantation at two high-volume centres. Figure 1 shows the flow chart of the study. Ten lesions in nine patients in whom a large SB was predilated were excluded to avoid the possible influence of this technique on SB patency following BVS implantation9. The remaining 140 patients with 190 lesions and a total of 346 SBs at risk constituted our study group. All enrolled patients had de novo lesions located in any native coronary artery. Exclusion criteria for treating with BVS were as follows: patients older than 70 years, vessel diameter >4 mm, very heavily calcified lesions, extreme tortuosity, patients with a contraindication to one year of dual antiplatelet therapy and patients in cardiogenic shock. Written informed consent for treatment and for data analysis was obtained from all patients.
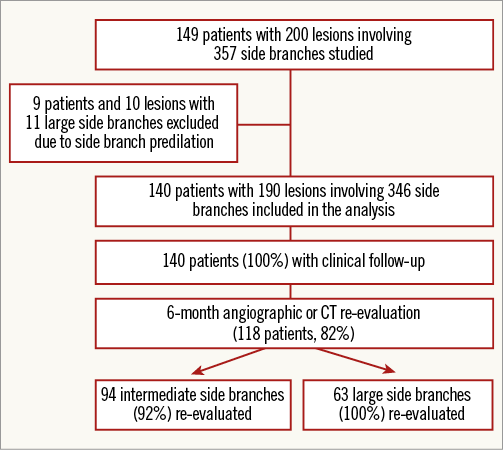
Figure 1. Study flow chart. CT: computed tomography
ANGIOGRAPHIC DATA
Quantitative angiographic studies (using CMS 7.1; Medis medical imaging systems bv, Leiden, The Netherlands) were performed pre and post percutaneous treatment to obtain the following measurements at the main vessel: diameter, lesion length, minimal lumen diameter and percentage of stenosis. The contrast-filled catheter was used as a reference.
All visible SBs originating within the scaffold segment or its 5 mm proximal or distal margins were included in the analysis10-12. A total of 346 SBs were identified. They were divided into three groups according to their visually estimated diameters. Therefore, SBs were small when the diameter was less than 1 mm, intermediate when the diameter ranged from 1 to 2 mm, and large when the diameter was larger than 2 mm. Significant damage at the SB origin was considered when the percentage of stenosis was >50% by visual inspection. Both classifications were determined off-line by the agreement of two expert interventional cardiologists. Lesions involving large SBs were assessed according to the Medina classification13.
Procedure
Large SBs were protected by a coronary guidewire before BVS implantation. After BVS implantation, the TIMI flow and ostium stenosis at the SB were evaluated. In patients with a TIMI flow <3 or a percentage of stenosis >75% by visual inspection, the SB was rewired, and balloon dilatation of the ostium was performed across the BVS. Based on our bench study, the use of balloons >2.5 mm and exceeding 8 atm of pressure was discouraged in order to avoid fractures of the BVS. In lesions involving only small or intermediate SBs, the BVS was implanted using the standard technique. Predilation of the main vessel was not mandatory, and post-dilation of the BVS was performed when non-apposition or non-expansion was observed by either optical coherence tomography (OCT) or intravascular ultrasound (IVUS). The use of OCT or IVUS was left to the discretion of the operator, except in cases of SB intervention after BVS implantation, in which the operators were encouraged to perform one of them.
The patients were pre-treated with dual antiplatelet medication. In the haemodynamic laboratory, they received a bolus of 100 IU/kg of intravenous unfractionated heparin. The administration of glycoprotein IIb/IIIa inhibitors was left to the discretion of the operator. After the procedure, all patients received 100 mg/day of aspirin indefinitely, as well as 75 mg/day of clopidogrel or 10 mg/day of prasugrel or 90 mg/12 hrs of ticagrelor for at least 12 months.
STUDY OUTCOMES AND DEFINITIONS
The assessment of SB patency was carried out by angiography immediately following BVS implantation. SBO was defined as a reduction in TIMI flow to grade 0 or 114. Serial determinations of troponin I and creatine kinase levels were performed before and every six hours after the procedure for the first 24 hours, and major adverse cardiac events (MACE) were recorded. MACE were defined as cardiac death, myocardial infarction (MI) and target lesion revascularisation. Periprocedural MI was defined as elevation of cTn values (>5 x 99th percentile URL) in patients with normal baseline values (≤99th percentile URL) or as a rise in cTn values >20% if baseline values were elevated and had been either stable or falling15.
FOLLOW-UP STUDY
The patients were closely monitored by telephone calls (at one and three months after the treatment) and scheduled visits (every six months during the first two years and yearly thereafter). In order to study the patency of significant SBs at follow-up, a coronary computed tomography scan (CT) was scheduled for every patient with jailed intermediate or large SBs six months after treatment. Angiographic re-evaluation was strongly recommended when symptoms or silent ischaemia were observed. Silent ischaemia was detected by performing exercise stress testing or myocardial perfusion imaging.
STATISTICAL ANALYSIS
Continuous variables are expressed as the mean±SD or median (interquartile range: IQ25-75) and were compared using the Student’s t-test or the Mann-Whitney U test. Categorical variables are presented as counts and percentages and were compared using the chi-square test or Fisher’s exact test, as appropriate. The unit of study in the univariate and multivariate analysis was the SB. Predictors of SBO were studied by multilevel regression using generalised estimating equations to correct for clustering of data. The model included random effects at the level of patients16. The goodness of fit of the model which contained the significant predictors of the effect was estimated by the corrected quasi-likelihood under the independence model criterion (QICC); the lower the QICC values, the better the fit of the model. The following variables were assessed in the univariate study: age, gender, smoker, hypertension, hypercholesterolaemia, diabetes, Q-wave MI, quantitative angiographic parameters at the main vessel (diameter, minimal lumen diameter, percentage of stenosis and lesion length), side branch size, stenosis >50% at the side branch ostium, number of BVS implanted/lesion, BVS diameter, ratio scaffold size/distal reference vessel diameter, implantation pressure, total scaffolding length and BVS post-dilation. Variables with a p-value ≤0.1 in univariate analyses and those considered clinically relevant were introduced into the model. Statistical analyses were performed using SPSS, Version 20 (IBM Corp., Armonk, NY, USA).
Results
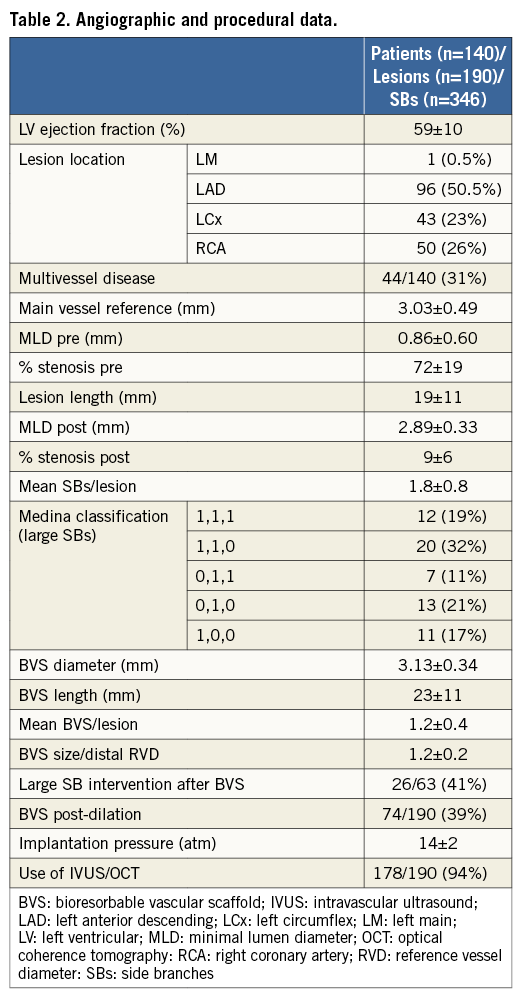
BASELINE CLINICAL, ANGIOGRAPHIC AND PROCEDURAL DATA
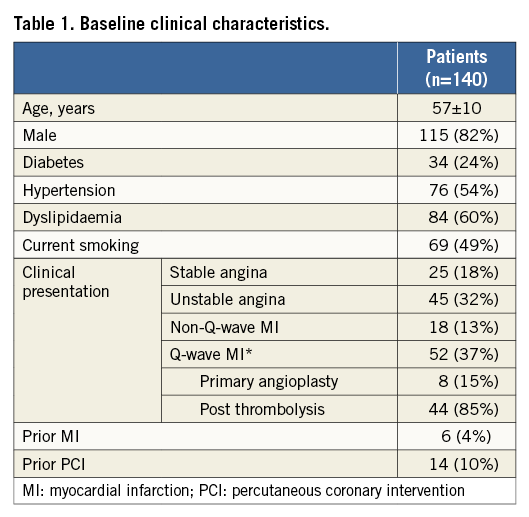
Baseline clinical data are summarised in Table 1. The mean age was 57±10 years. The majority of patients were male, had a high prevalence of risk factors and were admitted to the hospital due to acute coronary syndrome. Angiographic and procedural characteristics are detailed in Table 2. The most frequently treated vessel was the left anterior descending artery. In total, 346 jeopardised SBs were assessed. Based on SB diameter, 181 were small (<1 mm), 102 were intermediate (1-2 mm), and 63 were large SBs (>2 mm). Stenosis >50% at the SB ostium was observed in 15% to 19% of them (Table 3). After BVS implantation, additional intervention was required in 41% of the large SBs.
INCIDENCE OF SBO AND IN-HOSPITAL OUTCOMES
Immediately after BVS implantation, occlusions were documented in 31 of 346 SBs (9%). With respect to the size of the SBs, 22 of 181 small (12%), eight of 102 intermediate (8%), and one of 63 large SBs (1.6%) were occluded. However, the incidence of post-procedural SBO was much lower (19 [5.5%]) (Table 3). Seven SBs (64%) were spontaneously opened and four (36%) after balloon dilation. Regarding significant SBs (>1 mm), only two remained closed at the end of the procedure (Figure 2, Figure 3). One of these latter two, occluded in the context of a primary angioplasty, spontaneously recovered later and this was observed in the coronary angiography at three days (Figure 3). With regard to the location of the SBs, those which were occluded following BVS implantation originated within the scaffold segment (one small branch was located within an overlapped zone). No differences in the rate of SBO were found among the different clinical presentations (7% of SBO in stable angina, 11% in unstable angina, 9% when non-Q-wave MI was the clinical condition and 8% in Q-wave MI; p=0.8).
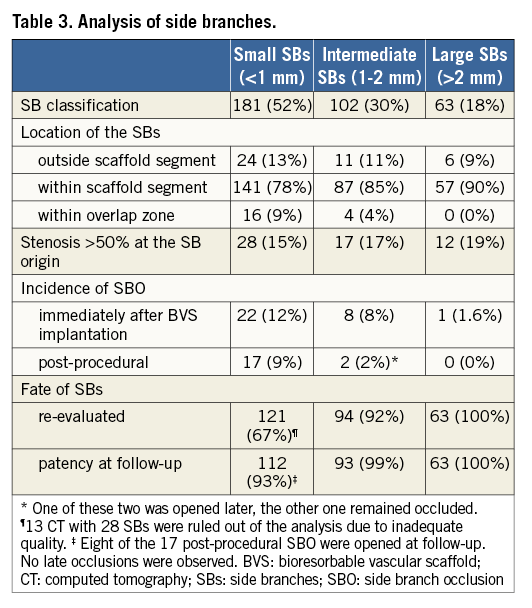
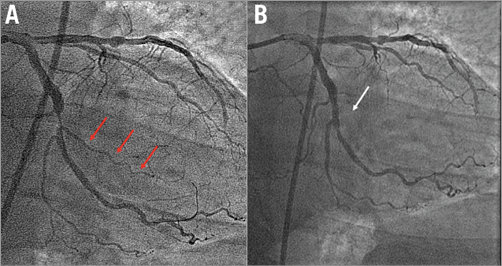
Figure 2. Intermediate SBO. Severe lesion in the left circumflex artery involving three side branches (A). Permanent intermediate SBO following BVS implantation (B). BVS: bioresorbable vascular scaffold; SBO: side branch occlusion
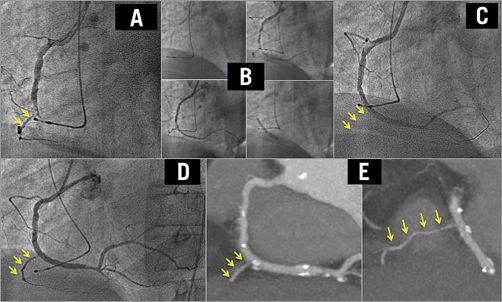
Figure 3. An intermediate SBO in a patient who underwent primary angioplasty due to an inferior AMI. The right coronary artery is occluded by a visible thrombus (A). A direct BVS was implanted (B) and a ventricular branch was lost (C). Three days later, the ventricular branch had been recovered (D). In the CT scan performed six months later, the ventricular branch remained patent (E). BVS: bioresorbable vascular scaffold; CT: computed tomography; SBO: side branch occlusion
Regarding clinical events, one patient suffered an acute MI due to BVS thrombosis 24 hours after the procedure. The patient was treated by balloon dilation. As clopidogrel resistance was detected, this was changed to prasugrel. Cardiac biomarkers were available in all of the patients: eight (6%) presented with significant troponin elevations in the range associated with an MI. However, in only one patient was this finding related to a permanent intermediate SBO, which was not rescued (Figure 2). A no-reflow phenomenon was the cause of the post-procedure troponin elevation in two patients, while in the remaining five patients any underlying angiographic mechanisms or no identifiable causes were observed. These patients did not have any symptoms or changes in the electrocardiogram. No other adverse clinical events were recorded.
FATE OF SIDE BRANCHES AND CLINICAL OUTCOMES AT FOLLOW-UP
The overall MACE rate at midterm follow-up (mean: 17±3 months, median: 17, IQ25-75: 15-19 months) was 5%. One patient died 58 days after treatment from definitive BVS thrombosis due to an interruption of dual antiplatelet therapy (he is not the one who had a thrombosis 24 hrs after the treatment). A total of 118 patients (84%) with 94 intermediate (92%) and 63 large (100%) SBs were re-evaluated by either CT (101 patients, 86%) or angiography (17 patients, 14%) 7±3 months after the procedure. Six (4%) patients presented restenosis at the main vessel. The restenosis was focal in the majority of patients (n=5, 83%) and located in the proximal segment of the main branch (n=4, 67%). Four (67%) patients were treated with new BVS, whereas the other two (33%) received a metallic stent. Another seven (6%) patients needed revascularisation due to progressing coronary artery disease in remote vessel areas. The remaining patients continue to be free of symptoms after a mean of 17 months of clinical follow-up (no patients were lost to follow-up). With respect to the significant SB patency at follow-up, no cases of late SBO were documented (Figure 4). Only the intermediate SB which was occluded and not recovered at the index procedure (Figure 2) remained closed six months later. The rest of the SBs which were occluded immediately after BVS implantation and afterwards recovered were patent at follow-up (Figure 3, Table 3).
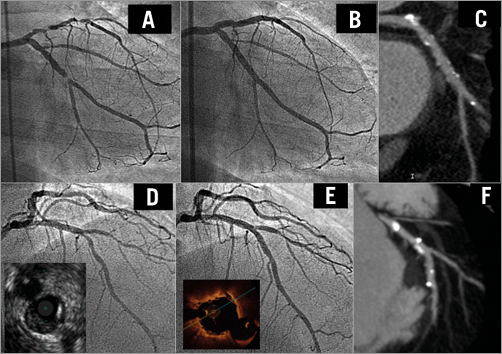
Figure 4. Immediate and follow-up patency of large SB covered by BVS. A severe bifurcation lesion (1,0,0) in the circumflex artery (A). A large side branch was covered by the BVS, with excellent immediate (B) and follow-up results (C). A bifurcation lesion (0,1,0) in the left anterior descending artery assessed by IVUS (D). A BVS was implanted covering the first diagonal, with a very good result noted by angiography and OCT (E). At six months, the CT demonstrated the excellent status of both branches (F). BVS: bioresorbable vascular scaffold; CT: computed tomography; IVUS: intravascular ultrasound; OCT: optical coherence tomography
PREDICTORS OF SBO
The multivariate predictors of SBO are shown in Table 4. Multilevel regression analysis identified the following two factors as independent predictors of post-procedural SBO: SBs <1 mm (OR 2.06, 95% CI: 1.08-4.63; p<0.05) and stenosis >50% at the SB’s origin (OR 17.22, 95% CI: 7.79-38.10; p<0.01).
Discussion
The present study assessed the incidence of occlusion of SBs of different sizes immediately after BVS implantation, as well as its clinical impact and the fate of jailed SBs at midterm follow-up. We found that the global incidence of SBO was 9% and significantly lower if post-procedural SBO is considered (5.5%). This phenomenon was essentially due to small SBs (<1 mm). Behaviour was more favourable in larger SBs, with only one case of occlusion noted in a vessel larger than 2 mm. In addition, the impact of occlusion on immediate outcomes was very low, and these promising results were maintained at midterm follow-up. To the best of our knowledge, this is the first study focusing on intermediate and large SBs, including true bifurcation lesions, covered by a BVS.
SBO AFTER METALLIC MAIN VESSEL STENTING AND THE INFLUENCE OF STRUT THICKNESS
There has been constant concern about the risk of SBO since the beginning of the stent era, and lesions involving SBs have been recognised as a potential source of acute complications. Several studies have analysed the incidence of SBO with bare metal stents, as well as with drug-eluting stents (DES). The reported rates of SBO have ranged from 7% to 19% among different studies. This variability may be related to different factors, such as SB size, ostial disease, presence of a thrombus-containing lesion at the main vessel and the type of stent platform used17-25. The thickness of the struts may also play a role in the incidence of SBO. Lansky et al10 compared the incidence of SBO in patients treated with everolimus-eluting stents (EES) (strut thickness 89 µm) with that observed in patients treated with paclitaxel-eluting stents (PES) (strut thickness 148 µm). Post-procedural SBO was documented in 2.7% of the SBs in the EES group and 4.3% in the PES group (p=0.06). Popma et al11 compared the patency of SBs in patients treated with zotarolimus-eluting stents (ZES) (strut thickness 96 µm) with that observed in patients treated with PES. Post-procedural SBO occurred less frequently in patients treated with ZES (2.2%) than in patients treated with PES (4.0%; p=0.032). Both studies suggested that strut thickness is a potential contributing factor in SBO.
SBO AFTER BVS IMPLANTATION
Focusing on the BVS, the greater thickness of the struts (157 µm), compared to the new DES, may be a factor influencing SBO. Muramatsu et al12 compared the incidence of SBO between patients treated with BVS and EES. Post-procedural SBO was observed more frequently in the BVS group (6%) than in the EES group (4.1%, p=0.09), but relying heavily on SBs ≤0.5 mm in diameter. In the present study, the global incidence of SBO was also higher than that reported in second-generation DES. In line with the previous study, the incidence of SBO was due primarily to the occlusion of SBs <1 mm, as well as to severe damage at the ostium. Additionally, the vessel wall area covered by the BVS strut is twice the surface area covered by the EES (26% for BVS vs. 12% for EES). Both findings reinforce the theory that small SBs are more likely to be compromised by the BVS, as it is more likely to cover the SB ostium fully.
SBO AND THE INCIDENCE OF ACUTE MI
SBO has been recognised as a factor in the development of periprocedural MI7,10-12,20,22,23. However, in this study, only one patient had a non-Q-wave MI as a consequence of permanent occlusion of an intermediate SB (Figure 2). In another patient, an intermediate ventricular branch also remained occluded at the end of the procedure. However, this took place in the context of a primary angioplasty, and therefore it is difficult to determine its real clinical repercussion (Figure 3). In the remaining patients with occlusion of significant SBs, the flow was rapidly restored either spontaneously or by balloon dilation at the same procedure. Thus, in these cases, no clinical sequels were observed. Moreover, the majority of the SBs occluded by the BVS were very small, and therefore the clinical impact on periprocedural myocardial damage was minimal.
FATE OF SIDE BRANCHES COVERED BY A BVS
There are insufficient data about the behaviour of struts covering the SB ostium at follow-up. Okamura et al26 showed one case in which the BVS struts were replaced by a neointimal membranous neocarina at the ostium of the diagonal branch two years after the original implantation. More recently, Onuma et al presented a small series addressing the fate of small SB ostia jailed by a BVS scaffold at six, 12, 24 and 36 months after implantation. Three-dimensional OCT analysis showed that the area free of struts at the SB ostium remained unchanged at six months, whereas at 12 and 24 months it was reduced due to the growth of tissue covering the struts. Afterwards, the ostium area increased due to the reduction of the neointima and the creation of a neocarina. However, these OCT findings were not related to any clinical consequences. There are some differences with respect to our study. On the one hand, a non-invasive imaging technique (CT) was routinely used to assess the fate of significant SBs in patients free of symptoms. Given the absence of metallic structures in the main vessel, the patency of the SBs was adequately visualised by CT in all patients27. We focused on re-evaluating the intermediate and large SBs, which have more interest from a clinical point of view (Figure 3, Figure 4). On the other hand, the additional intervention, when required, at these SBs through the BVS struts most likely modified the behaviour of the struts covering the SB ostia, avoiding the formation of a neointimal bridge. All SBs included in our study were patent at the six-month evaluation, regardless of whether they were treated. However, a longer follow-up period may be required in order to establish the safety of covering significant SBs and to assess the influence of manipulating the BVS after implantation in the main branch.
Limitations
The assessment of SB sizes, as well as the incidence of stenosis at the SB ostia, was performed by visual inspection. Mistakes in the classification of borderline SBs may have occurred. In order to avoid this problem, agreement between two expert interventional cardiologists was mandatory. On the other hand, an independent core laboratory was not used. Finally, the protective effect of the wiring in large SBs cannot be excluded, as it may have resulted in the low rate of large SBO.
Conclusions
Covering SBs with a BVS seems to be a safe procedure. The overall SBO rate, assessed immediately after BVS implantation, was 9% (the post-procedural rate was 5.5%), slightly higher than that reported with the latest-generation DES. However, this was closely related to SB size, with 1.6% of occlusions occurring in SBs larger than 2 mm in diameter. Additionally, the impact on clinical outcomes was very low, and these favourable results were maintained at short and midterm follow-up. However, long-term studies are needed to confirm these findings.
| Impact on daily practice The present manuscript analysed the incidence of immediate SBO after BVS implantation and its clinical impact. The analysis included SBs of different sizes. The main findings of this study focus on two points. First, intermediate and large SBs were included. Looking at the data, it seems safe enough to cover significant SBs with BVS, as the rate of occlusion was essentially due to small branches with irrelevant clinical impact. Second, it adds novel information about the fate of such SBs at follow-up. The behaviour of these SBs was very favourable, with no case of late occlusion observed in the jailed significant SBs that were re-evaluated. |
Appendix
CORONARY CT ANGIOGRAPHY PROTOCOL
Coronary CT angiography was performed using a 64-slice scanner (LightSpeed VCT; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). To reduce radiation exposure, a prospective acquisition of the images was performed, using the GE SnapShot pulse technique. Scan parameters included: slice acquisition 64×0.625 mm, gantry rotation time 350 ms, tube voltage (100-120 kV) and tube current (650-800 mA). The CT data were analysed on a dedicated workstation (Advantage Windows 4.5). For visualisation of the scaffold and the SBs, curved multiplanar reformations and maximum intensity projection were performed, selecting the best angle or perspective for better vessel analysis. Cross-sectional views of the vessel were also reconstructed at ~1 mm longitudinal steps, including the 5 mm proximal and distal to the device, using the platinum indicators as landmarks. BVS and SB patency was assessed visually. Patency was assumed if the contrast enhancement within a scaffold segment, distal to the BVS and in the SBs, was similar to the proximal coronary or aortic contrast enhancement.
Conflict of interest statement
The authors have no conflicts of interest to declare.

