Abstract
Aims: Our aim was to address the combined influence of myocardial perfusion defects and left ventricular ejection fraction (LVEF) on outcome with coronary revascularisation in stable CAD patients.
Methods and results: Of 527 patients with ischaemia by myocardial perfusion scintigraphy, 343 had medical therapy (Med) and 184 revascularisation (Revasc). During 5.3 years of follow-up, there was no intergroup difference in rates of death/myocardial infarction. Propensity score adjustment demonstrated a benefit of Revasc over Med with large defects (>14% of the myocardium), marked ischaemia (>10% of the myocardium), or LVEF <50%. However, defect size, ischaemia, and LVEF were correlated. In multivariate models, the Med versus Revasc hazard ratio (HR) was 4.06 times larger for LVEF <50% than for LVEF ≥50% (p=0.04) and 3.01 times larger for marked compared to mild/moderate ischaemia (p=0.11), whereas the effect of large compared to small/moderate defects vanished when adjusted for LVEF and ischaemia (HR=1.01, p=0.99). Considering the outcome difference as a function of both LVEF and ischaemia, we found no advantage or even a disadvantage of revascularisation in patients with mild/moderate ischaemia and preserved LVEF.
Conclusions: A benefit of revascularisation was found only in case of marked ischaemia or LVEF <50%. For treatment triage, both perfusion parameters and LVEF should be considered.
Introduction
Efforts on how to optimise treatment of stable coronary artery disease (CAD) focus on identifying patients who would benefit from revascularisation in addition to guideline-based medical therapy. An invasive strategy may reduce angina but has not shown superiority in decreasing death or non-fatal myocardial infarction (MI)1,2, regardless of ischaemia by functional imaging3.
Data from observational studies within the field of nuclear cardiology have indicated that a certain amount of myocardial ischaemia is crucial for the patient to gain a survival benefit from revascularisation4-7. Since the prognostic value of myocardial perfusion scintigraphy (MPS) is enhanced by considering also the left ventricular ejection fraction (LVEF)8,9, statistical modelling has been proposed to elucidate the combined role of perfusion and LVEF10,11. However, regarding treatment-specific outcome, reports on the interplay between ischaemia and LV function by MPS are sparse.
In extension of registry-based studies on the predictive value of MPS, we focused on outcome with respect to hard events, primarily all-cause death (ACD) or MI, in stable CAD patients with inducible ischaemia to assess the influence of defect size, ischaemia, and LVEF on the benefit from coronary revascularisation compared to medical therapy.
Methods
STUDY POPULATION AND DESIGN
From a consecutive series of 2,157 MPS performed during the period 2002-2007 at Odense University Hospital for suspected or known stable CAD, we followed 527 patients with reversible (n=324) or mixed defects (n=203). Results were analysed for all patients and subsets undergoing early revascularisation (Revasc) or receiving medical therapy (Med). Early revascularisation was defined as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within 180 days from MPS, while if revascularisation was performed >180 days later it was termed late revascularisation. The trial design and methods were published previously12.
MPS
MPS was performed as single photon emission computed tomography (SPECT) with technetium-99m sestamibi using a standard maximum exercise test or pharmacological stress by adenosine, dipyridamol, or dobutamine. As the original reports of the scans were often rather descriptive and made by different observers, we decided to reread all scans. Hence, in this study all scans showing ischaemia were interpreted semi-quantitatively by one experienced reader (A. Johansen) without knowledge of the clinical outcome. The extent and severity of perfusion defects at stress imaging and of reversible perfusion defects representing ischaemia were converted to percentage myocardium with stress defects being categorised as small (5-9% of the LV myocardium), moderate (10-14%), or large (>14%), and ischaemia as mild (3-5% of the LV myocardium), moderate (6-10%), or marked (>10%)6,13. In the early study period non-gated acquisitions were used. Later, gated studies were used with at-rest LVEF being available in 316 patients (60%), and post-stress LVEF in 297 (56%).
STATISTICS
Intergroup differences in continuous variables were tested by the Wilcoxon rank-sum test; frequencies were compared by Fisher’s exact test or the chi-squared test. Correlation between ordinal variables was assessed by means of Spearman’s rho. Since patients were not randomised to revascularisation, a logistic regression model was used to provide propensity scores representing the probability of patient referral to one therapy versus another14. Clinical and scintigraphic variables, and in patients with catheterisation angiographic results also, with a significantly uneven distribution or a significant association after adjustment for defect size, ischaemia, angina score according to the Canadian Cardiovascular Society (CCS), and number of stenotic arteries, were included in the logistic model.
Propensity score-adjusted Cox models were used to estimate the outcome difference between Med and Revasc as a hazard ratio (HR). The influence of a covariate on the outcome difference was expressed as a relative hazard ratio (RHR). For dichotomous covariates, an RHR of 1 signifies equal outcome differences between Med and Revasc for both values of the factor, i.e., the HRs found in the two subgroups defined by the covariate are identical. Specifying one of the two possible values of the dichotomous covariate, an RHR >1 means a poorer prognosis with Med than with Revasc for the subgroup defined by this specific value of the covariate; an RHR <1 means the opposite. RHRs were estimated based on a Cox model with corresponding interactions. This approach allows incorporating continuous covariates where an HR describing the outcome difference between Med and Revasc can be estimated for each value of the covariate. This also provides a means of determining breakpoints, i.e., the value of a covariate where the HR changes from values larger than 1 to values smaller than 1.
The primary endpoint was ACD or MI, whereas secondary endpoints were ACD, cardiac death (CD)/MI, and ACD/MI/late revascularisation. In the analyses of the composite endpoints only the time until the first event was considered. Follow-up continued until the date of the respective event, emigration, or end of follow-up period (31 December 2011). When analysing death and/or MI, patients were censored in case of late revascularisation before the event of interest in order not to bias the natural course. When analysing CD, patients were censored in case of death from other causes.
Incidence rate comparisons were carried out according to Boyd and Radson15. The significance level was set at 5%. Statistical analyses were performed using Stata Statistical Software, Release 12 (StataCorp LP, College Station, TX, USA).
Results
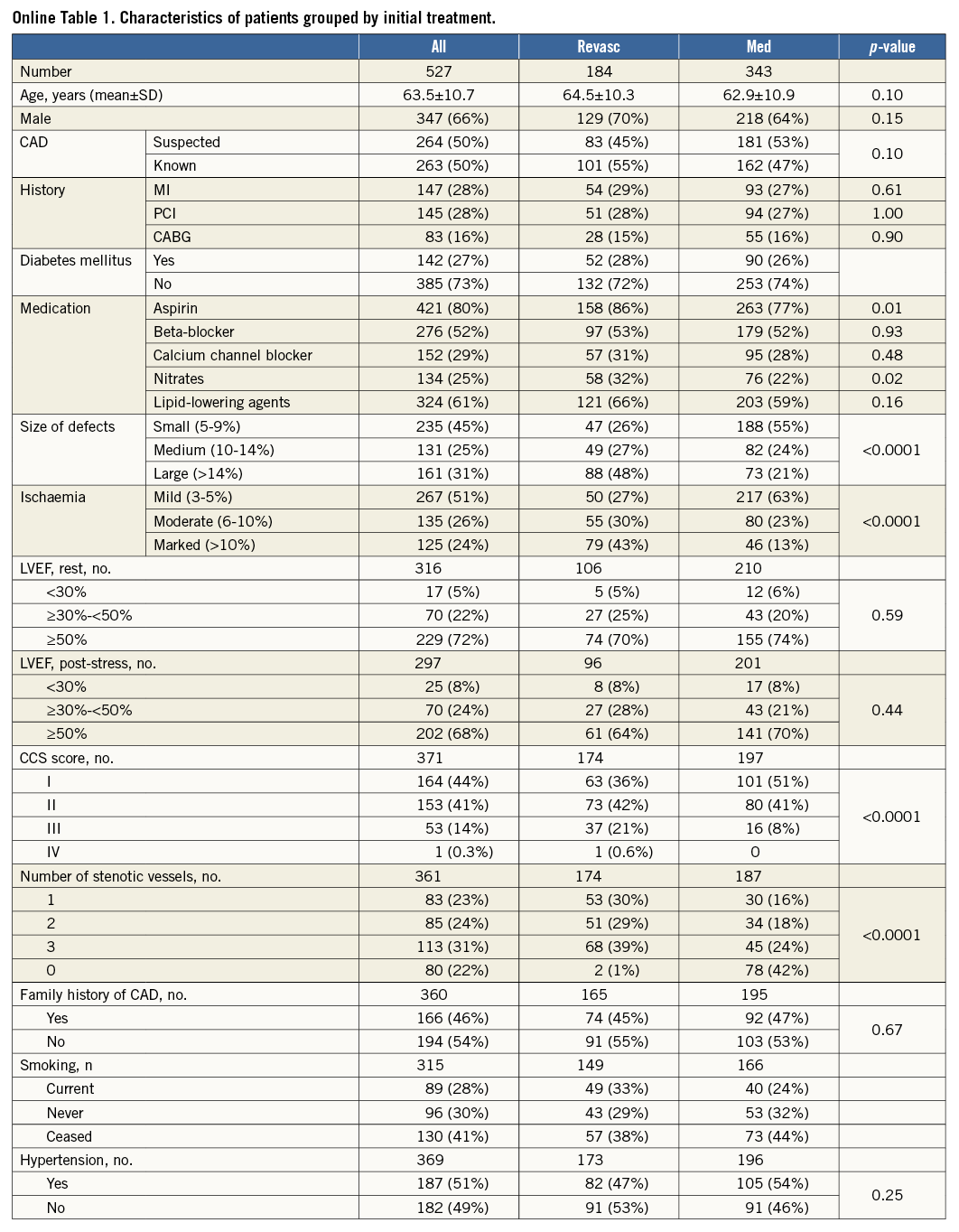
Out of 527 patients with ischaemia, 184 (35%) underwent early revascularisation. Online Table 1 displays clinical and scintigraphic characteristics as well as angiographic data for patients with a temporally related catheterisation (±6 months). Treatment groups were similar in many respects but differed by defect size and ischaemia (both p<0.0001). More Revasc patients used aspirin or nitrates (p=0.01 and p=0.02, respectively), had severe angina, and had more coronary arteries affected (both p<0.0001). There were no intergroup differences in LVEF.
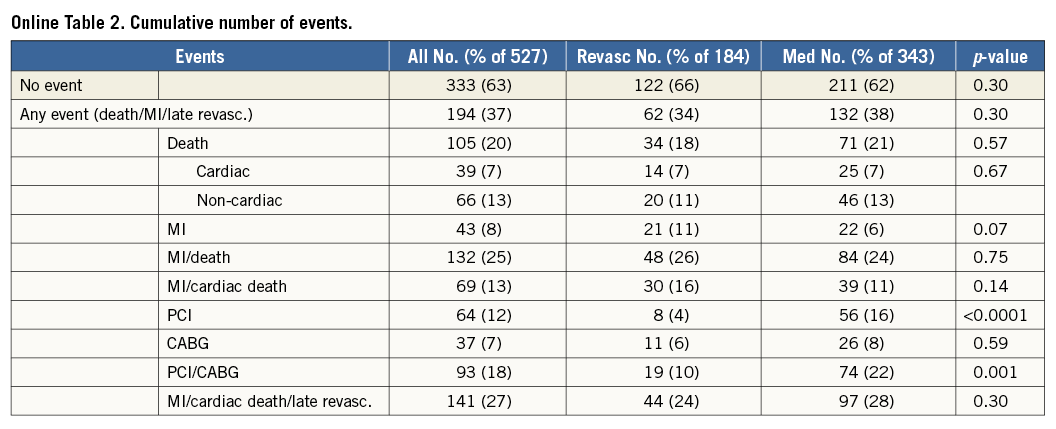
Median (range) follow-up was 5.3 (0.24-9.99) years. Overall, 333 patients (63%) were event-free survivors and 105 (20%) died, independently of initial treatment (Online Table 2). The number of MIs tended to be higher in the Revasc group (11% versus 6%, p=0.07), whereas the number of late PCI procedures was higher in the Med group (16% versus 4%, p<0.0001) (Online Table 2). The incidence rate of ACD/MI did not differ by group, being 5.1% in the Med group and 4.9% in the Revasc group, respectively, p=0.79. The incidence rates of secondary endpoints also did not vary by group (data not shown).
The propensity score model included age, gender, CAD (known versus suspected at the time of MPS), previous MI, previous PCI, previous CABG, diabetes, use of medication, defect size, and amount of ischaemia. When angiographic variables were available, the CCS score and the number of arteries affected were also included.
Propensity score-adjusted HRs (CI) for the Med versus Revasc difference were computed for subgroups defined by defect size, ischaemia, and LVEF for all four outcomes: these are shown in Figure 1. For the primary endpoint, stratification according to perfusion defects showed that only in case of large defects or marked ischaemia was medical therapy associated with a significantly higher risk than was revascularisation. Regarding functional values, HRs in patients with LVEF <50% were 3 to 6, signifying a very distinct survival benefit with revascularisation, whereas HRs in patients with LVEF ≥50% were close to 1. The other endpoints showed similar results.
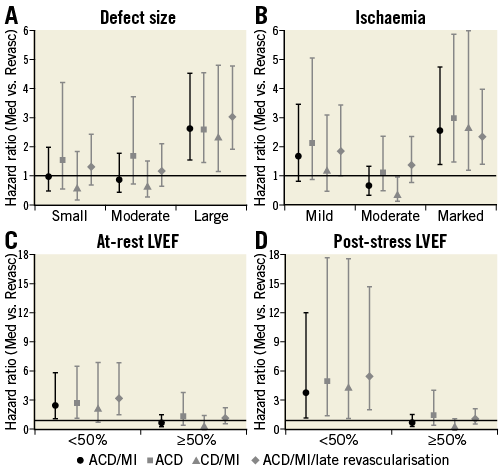
Figure 1. Hazard ratios in subgroups. Relative risk with Med vs. Revasc for the primary endpoint (black circles). Similar numbers were found for secondary endpoints (grey squares, triangles, and rhombs). For the primary endpoint, testing equality of the hazard ratios across the subgroups yielded p-values of <0.02 for all variables shown.
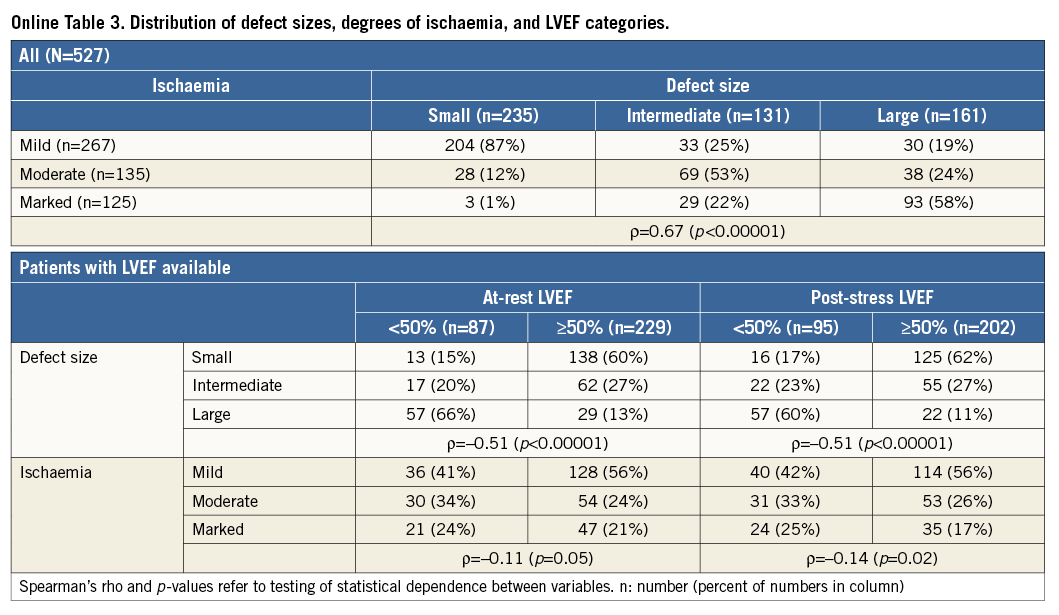
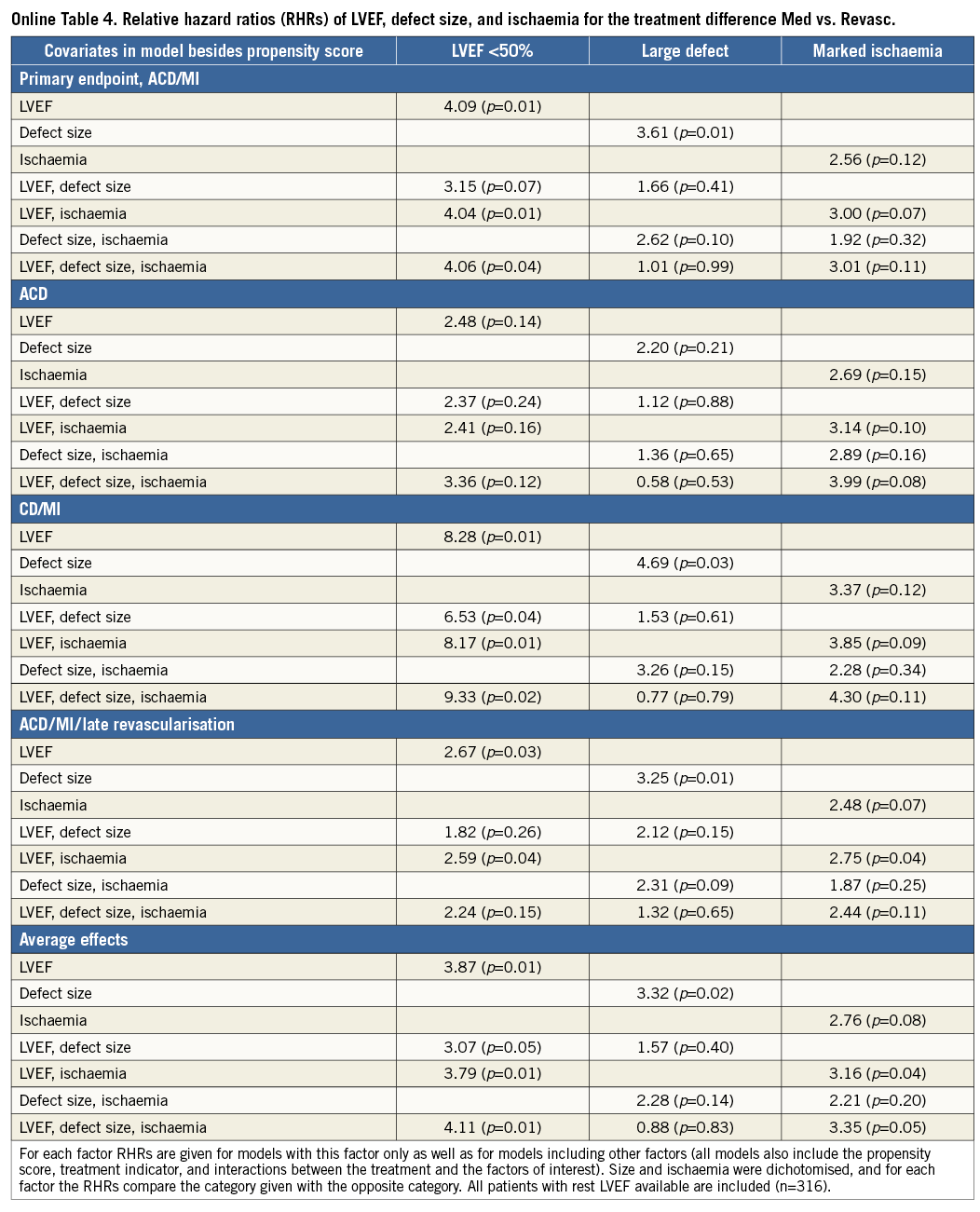
Defect size and ischaemia were correlated with marked ischaemia found predominantly in case of large defects, with small defects being associated primarily with mild reversibility (Online Table 3). Furthermore, defect size was correlated with LVEF, with 2/3 of those with large defects having LVEF <50% and patients with large defects constituting the majority of the group with reduced LVEF. For ischaemia, the correlation with LVEF was less pronounced (Online Table 3). RHRs describing the effect of these three factors on the outcome difference for various combinations of adjustment are shown in Online Table 4. When only one factor was included in the model, moderate effects were observed for all three factors. However, with adjustment for LVEF the effect of defect size tended to vanish, whereas the effect of ischaemia slightly increased. In the models with all three factors the effect of defect size was always close to unity. These results applied to all endpoints, and, when averaging over all endpoints, LVEF and ischaemia were significant predictors of the outcome difference in a model with these two variables and still borderline significant in the model with all three factors, with low LVEF and marked ischaemia associated with increased risk of Med over Revasc. For the single outcomes, in particular for the primary endpoint, marked ischaemia reached p-values between 0.04 and 0.11 when adjusted for LVEF or LVEF and defect size. In Online Table 4, data are given for at-rest LVEF. For models including post-stress LVEF instead, LVEF was also significantly predictive of the effect of revascularisation, while the effects of defect size and ischaemia were of similar magnitude and insignificant.
Kaplan-Meier curves based on a propensity score-adjusted Cox model comparing Med to Revasc in combined dichotomised ischaemia and at-rest LVEF categories are shown in Figure 2 for the primary endpoint. Similar graphs were made for the other endpoints as well as for post-stress LVEF categories. These curves illustrate that, with LVEF ≥50% and mild/moderate ischaemia (Figure 2A, n=182 [58%]), there was never a benefit from revascularisation. In some cases the survival estimate for the Revasc group was actually below that for the Med group, indicating a detrimental effect of revascularisation. With LVEF ≥50% and marked ischaemia (Figure 2B, n=47 [15%]), curves often ran close together with a minor advantage for Revasc. With LVEF <50% (Figure 2C, Figure 2D, n=87 [28%]), estimated survival for the Revasc group was clearly above that of the Med group.
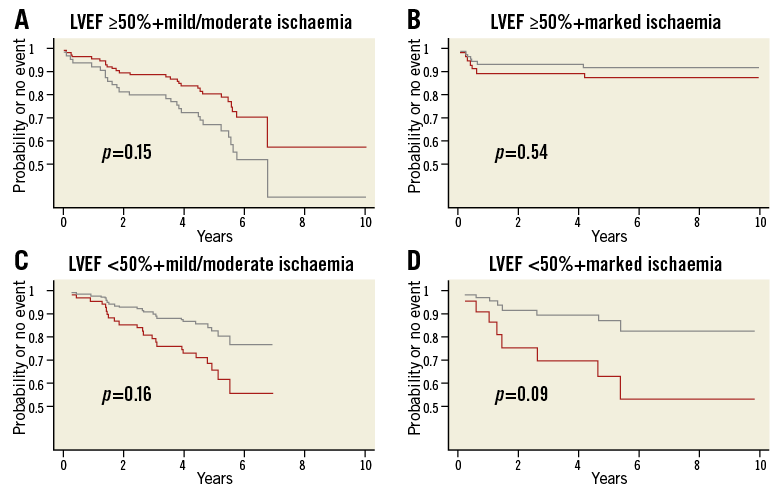
Figure 2. Model-based time-to-event curves for the endpoint ACD/MI. A) & B) At-rest LVEF ≥50%; C) & D) at-rest LVEF <50%. A) & C) Mild/moderate ischaemia; B) & D) marked ischaemia. Red line, Med; grey line, Revasc. Curves are based on a Cox model including the propensity score and the treatment indicator. The models were fitted separately for the four subgroups. Graphs were similar for the other endpoints.
From a model for the outcome difference with LVEF as a continuous factor and ischaemia dichotomised, the Med versus Revasc HRs are depicted in Figure 3 for different outcomes. Basically, HR was >1 in case of marked ischaemia, indicating a higher risk with Med than with Revasc, independently of LVEF. In case of mild/moderate ischaemia the HR depended on LVEF with HR being >1 for LVEF below 40-50%. Only in case of preserved LVEF and mild/moderate ischaemia was HR for the Med versus Revasc comparison <1. These results applied also when post-stress LVEF was used instead. In case of mild/moderate ischaemia, breakpoints (CI) averaged over all endpoints were: at-rest LVEF 45% (25, 65), post-stress LVEF 49% (37, 62).
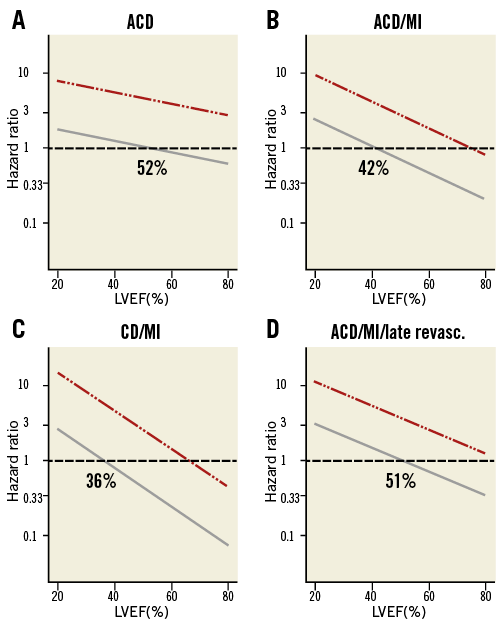
Figure 3. Model-based hazard ratios for the outcome difference Med vs. Revasc. Hazard ratios for continuous values of at-rest LVEF (abscissa) and dichotomised values of ischaemia (solid grey line, mild/moderate; dash-dotted red line, marked) for different endpoints. Numbers indicate breakpoints in case of mild/moderate ischaemia. Curves were similar for post-stress LVEF.
Discussion
With reversible perfusion defects, the amount of ischaemia and LVEF seem to be independent predictors of the benefit of Revasc over Med, although the independent effect of marked ischaemia reached significance only when averaging over all outcomes. However, in any case, our results suggest that the size of the defect is not an independent predictor. Actually, defect size correlated with LVEF, and, thus, in univariate analyses size was associated with the Med versus Revasc difference. This correlation is to be expected, since reduced oxygenation following from reduced perfusion also affects the contractile function of the myocardium. Hence, when assessing the effects of perfusion parameters and LVEF on outcome, the interrelationship between these factors should be taken into consideration.
The combined impact of ischaemia and LVEF on outcome after revascularisation is illustrated in Figure 3. In case of mild/moderate ischaemia, the HR for the outcome difference crossed the equipoise, i.e., patients with preserved LVEF were better off without revascularisation. Considering CIs for breakpoints, we could demonstrate a negative effect of revascularisation with LVEFs above 60%; the breakpoint estimates themselves supported a limit of 50%. These results applied to all endpoints.
Whom to revascularise?
Many factors may have an effect on the benefit of revascularisation16. There is a growing perception of the importance of a function-based rather than a purely structure-based approach to this issue9,17,18. One such function-based approach is MPS demonstrating compromised myocardial perfusion and its functional consequences. Observational nuclear cardiology trials have shown that at least 10% of the LV myocardium should be ischaemic for the patient to gain a survival benefit from revascularisation4-6,17,19,20. The same amount is required to achieve improvement in symptoms and exercise capacity21,22. Ischaemia is a more important predictor of outcome according to treatment strategy than the extent and severity of fixed perfusion defects4. Hitherto, these results were not confirmed in randomised controlled trials (RCTs) showing no overall prognostic advantage of revascularisation over optimal medical therapy or, at best, a reduced number of late revascularisations1, irrespective of the baseline ischaemia burden23. Several possible reasons for this have been proposed, including the ischaemia-reducing effect of modern drugs24,25. Generally, in the RCTs concerned, variations in LVEF were not taken into account.
The observation that a survival benefit of coronary revascularisation is only to be expected with a reduced LVEF has been known for decades26,27, and, typically, a cut point of 50% was used. However, the combined effect of ischaemia and LVEF on treatment-specific outcome has been scantily addressed so far. Hachamovitch et al in a pragmatic trial described that LVEF and ischaemia added to each other for the prediction of CD, and that patients with a lower LVEF had a greater absolute benefit from revascularisation; yet, they only included interaction between ischaemia and revascularisation in their reported model and concluded that only ischaemia identified patients with survival benefit from revascularisation28. In another work on a subgroup of patients >75 years it was stated explicitly that no MPS data other than ischaemia interacted with ACD in treatment groups19. Contrary to their findings that ischaemia was superior to LVEF in predicting which post-MPS clinical strategy would enhance short-term (2.8 years) patient survival28, we found that LVEF was a strong independent predictor. Furthermore, Hachamovitch’s group concluded that patients with limited ischaemia – regardless of LVEF – have a better survival with medical therapy, and that in patients with LV dysfunction but without inducible ischaemia there is no therapeutic benefit with revascularisation28. Our results are more differentiated and support recent findings that with a reduced LVEF the benefit from revascularisation is not limited to patients with certain amounts of ischaemia29.
Strengths and weaknesses
The choice of endpoints in this area of research is not clear-cut. Typically, hard cardiac events were reported4,7,28, and some authors included cardiac symptoms requiring hospitalisation6 or stroke30, but, more recently, rates of ACD were given5. We considered different hard endpoints, and, fundamentally, our results applied to all of them, as illustrated in Figure 3.
We included only patients with some degree of reversible perfusion defects, since only in these is the question of whom to revascularise relevant. The price of doing this was a smaller cohort, limiting the power. Furthermore, our definition of marked ischaemia as >10% may imply an underestimation of the RHR describing the effect of ischaemia on the outcome difference compared to other studies, which have categorised involvement of 10-20% of the myocardium as moderate ischaemia and >20% as marked4. However, in the COURAGE Nuclear Substudy 2, moderate to severe ischaemia was also defined as ≥10% LV ischaemia31.
MPS was performed as part of routine diagnostic work-up, and treatment assignment post-MPS was a clinical decision also based on other examinations of the patients. This could explain why 2/3 of the patients with ischaemia did not undergo coronary revascularisation in the first place. As in other effectiveness studies, there was no systematic approach to medical therapy in the Med or the Revasc group; patients were assumed to receive care of contemporary standard without knowledge of their compliance. An inherent limitation of a registry-based study using propensity scores is the possibly insufficient adjustment for all data influencing referral. Patients in the Revasc group might have been sicker, or, in case of severe comorbidity, revascularisation might have been abandoned. Post-MPS catheterisation referral is typically directly related to the amount of abnormally perfused myocardium32,33 besides a variety of clinical factors, most importantly angina28. We too found more abnormal perfusion and higher CCS scores in the Revasc group. Even if we failed to adjust completely for all confounders, it is unlikely that this had a substantial effect on our results, as we found very large treatment differences ranging from HRs ≥3 down to ≤1/3.
Conclusions
In patients with stable CAD and ischaemia by MPS assigned to standard medical therapy, additional revascularisation may improve hard event outcome. We demonstrated that the benefit from revascularisation depends on both LVEF and the amount of ischaemia. The latter is of particular importance in patients with preserved LVEF, while reduced LVEF per se signifies a survival benefit from revascularisation in case of any amount of ischaemia. Whereas the limit of 10% ischaemia needed to gain prognostic benefit of revascularisation has been proposed for some years, we could support the often used categorisation of LVEF below and above 50% as an additional criterion. In patients with preserved LVEF and limited ischaemia – in our cohort constituting the majority of patients – medical treatment should be the choice. Larger studies are necessary to estimate with a higher precision the boundary line between patients to be offered revascularisation or medical treatment, which may also depend on further patient characteristics such as age, gender, or diabetes.
| Impact on daily practice In the debate on appropriate treatment of patients with stable ischaemic heart disease, i.e., optimal medical therapy alone or in combination with coronary revascularisation, attention has been brought to the amount of inducible ischaemia, since observational studies have indicated a benefit from revascularisation only in case of >10% ischaemia. Myocardial perfusion scintigraphy provides estimates of both extent and severity of perfusion defects, reversibility, and LVEF. We demonstrated both ischaemia and LVEF to be independent predictors of treatment-specific outcome, and, consequently, treatment assignment should be based on both, i.e., revascularisation should be considered only in case of marked ischaemia or reduced LVEF. |
Funding
This work was part of a PhD project. The first author received a PhD scholarship from The PhD Research Fund at Odense University Hospital, Odense, Denmark.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data