Abstract
Aims: Our aim was to evaluate the effectiveness and safety of CenterCross/MultiCross devices to facilitate the crossing of chronic total occlusions in peripheral arteries.
Methods and results: This was a single-centre study in which 53 consecutive patients who were not amenable to initial attempts at crossing using standard guidewires underwent an attempt to recanalise chronically occluded infrainguinal peripheral arteries with MultiCross/CenterCross devices. The primary endpoint of interest was the ability to advance the guidewire beyond the chronic total occlusion (CTO) lesions with the use of these devices. Safety endpoints were freedom from bleeding, distal embolisation and vessel perforation, dissection or need for emergent surgical intervention. Popliteal artery and below lesions were the most commonly treated, comprising 89% of the total. The CTO lesions were crossed successfully in 92.4% of the cases within a relatively short time (5.5±3.5 minutes). There was no bleeding, dissection or need for emergent surgery and 98.1% and 96.2% of the patients were free from distal embolisation and perforations, respectively.
Conclusions: Our study demonstrated that MultiCross and CenterCross were effective and safe for recanalisation of peripheral CTO lesions which were not amenable to conventional guidewires. Further study is required to define the role of these novel devices in the treatment of complex lesions, particularly CTOs in patients with peripheral arterial disease.
Abbreviations
CTO: chronic total occlusion
PAD: peripheral arterial disease
TASC: TransAtlantic Inter-Society Consensus
Introduction
Peripheral arterial disease (PAD) affects about six to 12 million people in the United States of America and is associated with significant morbidity and mortality1. The symptoms of PAD include intermittent claudication, rest pain, tissue necrosis, ulceration, limb loss and death2. Up to 20% of the population of the USA above 75 years of age have some features of PAD. This prevalence is likely to increase with the aging of the general population3.
Surgical bypass and/or surgical endarterectomy have been the main therapeutic options for alleviating symptoms, preventing limb loss and improving quality of life of patients with severe PAD3. In fact, bypass surgery has been endorsed as the preferred treatment for CTO lesions by TransAtlantic Inter-Society Consensus recommendations (TASC II)4. However, surgery is associated with high morbidity and mortality, particularly as many patients with PAD have increased comorbid conditions, including (amongst others) concomitant coronary artery and cerebrovascular disease3,5. Endovascular treatment strategies, which are less invasive than bypass surgery, have evolved over the past 10 years and have increasingly become the revascularisation methods of choice for patients with severe PAD5. The advantages of endovascular therapies over peripheral bypass surgery are rapid recovery and an early return to daily activities, less morbidity, shorter hospital stay and lower costs3,5.
However, the “Achilles heel” of endovascular therapeutic procedures remains chronic total occlusion (CTO), which represents about 20-40% of lesions in patients with symptomatic PAD1. Not only do these procedures for CTO require significant time, radiation and contrast use, but also, more importantly, they are associated with suboptimal procedural success rates and greater complications2. Failure to recanalise CTO occurs in 20% of cases: such procedures are not uncommonly associated with higher rates of complications such as arterial dissection and/or perforation, distal embolisation and bleeding6. These shortcomings have resulted in increasing interest and investment from operators as well as industry, not only in evaluating new strategies and therapies, but also in developing new technology to increase procedural success rates and decrease complications. As a result, there has been a proliferation of strategies and devices designed to improve the procedural success and outcomes in these patients7.
One such recent innovation to facilitate crossing of CTO in PAD patients is the arrival of the CenterCross™/MultiCross™ devices (Roxwood Medical, Inc., Redwood City, CA, USA). We describe the initial experience with the use of these devices for the treatment of CTO at a single tertiary care centre.
Methods
PATIENT POPULATION
Between August 2014 and January 2015, 53 patients who were not amenable to initial attempts at standard guidewire crossing underwent an attempt to recanalise chronically occluded infrainguinal arteries using the MultiCross/CenterCross device at the St. John Hospital and Medical Center. An experienced operator with expertise in complex peripheral interventions performed all interventions. Informed consent was obtained from all patients for the procedure.
CENTERCROSS AND MULTICROSS DEVICES
The CenterCross device (Figure 1A) is designed to facilitate guidewire delivery through complex lesions, particularly through blockages in patients with PAD. The device comprises a resheathable, self-expandable nitinol scaffold that is 10 mm long and has an expanded diameter of 4 mm. It has a 3 Fr inner lumen, which accommodates either a 0.014” or a 0.018” microcatheter and guidewire, or a 0.035” guidewire. The device has a working length of 130 cm. The shaft diameter is 1.8 mm (0.071”) which is compatible with a 7 Fr guide catheter and a 6 Fr introducer sheath. The device is advanced proximal to the lesion where the scaffold deploys to centre and anchor, providing maximum support for the microcatheter and guidewire to navigate through the lesion. Once the guidewire penetrates and crosses the lesion, CenterCross is removed from the vessel, allowing the operator to perform angioplasty, atherectomy or stenting of the lesion over the guidewire used to cross the lesion.
The MultiCross device (Figure 1B) is similar to the CenterCross, but enables three separate 0.014” guidewires to be delivered through a similar platform comprising a resheathable, self-expanding nitinol scaffold. These lumens are anchored away from the vessel wall to facilitate intraluminal access. It has a working length of 135 cm with a shaft diameter of 1.8 mm (0.071”) which is compatible with a 7 Fr guide catheter and a 6 Fr introducer sheath. The scaffold is 10 mm in length with an expanded diameter of 4.7 mm. The MultiCross provides clinicians with greater options for wire escalation using multiple 0.014” guidewires simultaneously to cross the lesion. If the first guidewire meets resistance or is tracked into a subintimal plane, the operator may utilise a second or third 0.014” guidewire in the remaining lumens. Once the lesion is crossed, the operator leaves the crossing guidewire in the distal vessel, resheaths the MultiCross and removes it from the vessel, allowing the operator to use the wire across the lesion to perform angioplasty, atherectomy or stenting of the lesion over the guidewire used to cross the lesion. These devices can be used to facilitate the treatment of any CTO in the peripheral arteries and there are no device-specific exclusions.
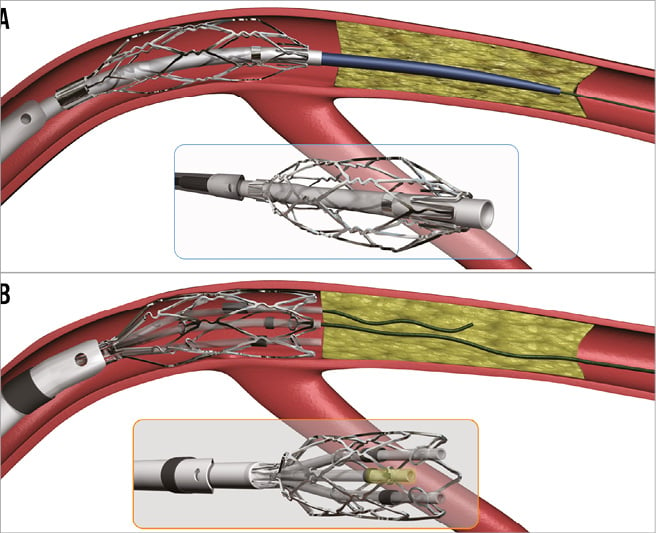
Figure 1. The CenterCross and MultiCross devices. A) Cartoon of the CenterCross device (Roxwood Medical, Inc.). B) Cartoon of the MultiCross device (Roxwood Medical, Inc.).
PROCEDURAL DETAILS
A contralateral retrograde or ipsilateral femoral access was obtained in patients with infrainguinal CTO. Intravenous unfractionated heparin was used to attain an activated clotting time >250 seconds. After attempts to use conventional wires to cross CTOs failed, CenterCross or MultiCross catheters were used to assist the advancement of a guidewire across the CTO. No other conventional CTO crossing devices were attempted before using CenterCross/MultiCross. CenterCross or MultiCross catheters were used to advance a guidewire across the CTO. Once this was achieved, various devices were used to treat the lesion including balloon angioplasty, directional or rotational atherectomy, drug-eluting balloon and/or stenting. Post-dilation of lesions was performed in most cases. Atherectomy was performed with the use of an embolic protection device to prevent distal embolisation. Non-ionic contrast was used during all procedures. All patients received intravenous hydration before and after the procedure, aspirin 325 mg before the procedure and thienopyridine after the procedure. Besides angiography, no other invasive imaging modality such as intravascular ultrasound was used before or after the intervention to guide and/or assess the results of the procedure. Groin closure was achieved using vascular closure devices or manual pressure. After the procedure, all patients were monitored overnight in a post-procedure observation unit and discharged the next day if stable.
DATA COLLECTION AND DEFINITIONS
Data were collected retrospectively for all patients and entered manually into an Excel database. The information gathered included patients’ demographics, comorbid conditions, medical treatments, lesion characteristics, procedure details, in-hospital complications and outcomes. Procedure details included the success of crossing, crossing time (time from the insertion of the device until the lesion was crossed), total contrast used, fluoroscopy time and procedural success. Occlusion was defined as 100% blockage without notable antegrade blood flow during angiography. Lesion calcification was defined using a qualitative method as heavy (or not) if densities involved both sides of the arterial wall prior to contrast injection1. Successful device crossing was attained when advancement of the guidewire through the CTO lesion was verified by angiography. Procedural success was defined as <30% residual stenosis in the absence of any dissection, perforation or distal embolisation. Bleeding was defined as a decrease in periprocedural haemoglobin of >3 g/dl. Contrast-induced nephropathy was defined as a 25% increase in creatinine from baseline within 48 hours after the procedure. Mortality was all-cause death in-hospital. Bleeding risk at baseline for all individual patients was estimated using a previously published model8.
STATISTICAL METHODS
Continuous variables are expressed as mean±standard deviation and median and 25th and 75th percentile. Categorical variables are presented as frequency counts and percentages.
Results
Baseline demographics and comorbidities are shown in Table 1. Of the 53 patients with PAD who underwent CTO intervention, the majority were male (71%). Patients’ mean age was 74 years and there was a high prevalence of comorbid conditions, including hypertension and hyperlipidaemia in almost everyone, and diabetes and coronary artery disease in three quarters of the patients. The majority of patients were smokers (70%). About three quarters of patients had had prior percutaneous peripheral vascular interventions, half had had prior peripheral bypass surgery and a quarter had had prior amputation. Almost all patients had symptoms at rest and over half presented with tissue loss.
Lesion location and characteristics are summarised in Table 2. CTO lesions in the popliteal artery and below were the most commonly treated vessels, comprising 89% of the total, whereas superficial femoral artery occlusions represented the minority, comprising only 11% of the treated lesions. The mean length of CTO lesions was long with an average of 154.7±49.3 mm. Heavy calcification was present in 21 of the 53 patients.
Procedure success was achieved in the vast majority of patients (92.4%) (Table 3). This was achieved in 20/21 patients (95.2%) with heavy calcification versus 29/32 patients (90.6%) without heavy calcification. The average crossing and fluoroscopy times were 5.5 and 27 minutes, respectively, with a mean contrast medium use of 121 ml. After successful CTO lesion crossing, directional and rotational atherectomy were the most commonly performed procedures. Only a small minority (n=8) of patients were treated with stent placement in our series.
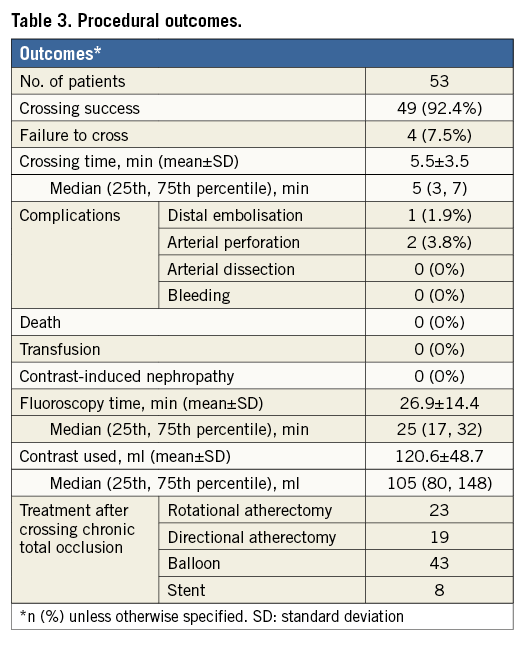
Procedural complications were low (Table 3): only two patients had guidewire perforation and one had distal embolisation - these were managed conservatively without any major adverse consequences. Bleeding, need for transfusion, vessel dissection, contrast-induced nephropathy and/or need for emergent peripheral vascular surgery were not observed in any patients.
Discussion
We present the initial findings of novel crossing devices (CenterCross and MultiCross) for infrainguinal CTO lesions. These devices were designed to facilitate crossing by securing the guidewire just proximal to the CTO lesion. We must emphasise that this is a preliminary retrospective report of interventions upon only 53 patients with infrainguinal CTOs. The results were encouraging in this small group of patients with successful crossing attained in 92% of patients at a relatively short crossing time of 5.5 minutes and low contrast use (mean=121 ml) and average fluoroscopy time (mean=27 minutes), despite very long lesions (mean lesion length of 155±49.3 mm). Additionally, qualitatively defined heavy calcification was not associated with device failure to cross the lesion. Finally, the procedures were completed with a low rate of complications (bleeding 0%, dissection 0%, perforation 3.8% and embolisation 1.9%), and no patient required emergent peripheral arterial surgery.
Multiple other devices have been used for crossing CTO lesions with varying success rates1,2,6,9-16 (Table 4) and complication rates1,2,6,9-16 (Table 5). None of these devices was compared directly with another in a randomised fashion to evaluate their relative efficacy. Similarly, direct comparisons cannot be made between the CenterCross/MultiCross system and the other currently available devices reported in Table 4 and Table 5, as the patient populations, procedural details, and lesion characteristics may not be similar.
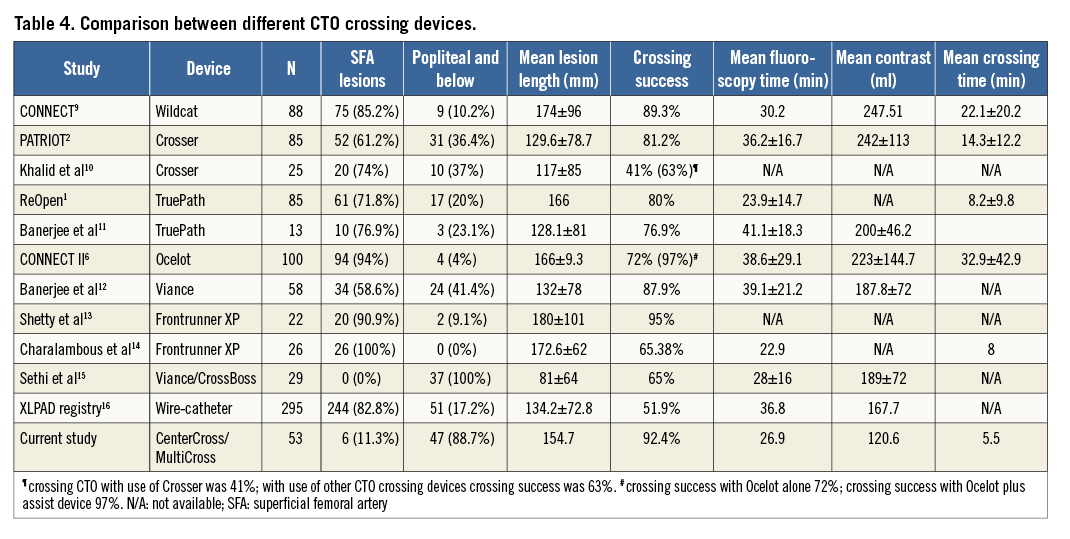
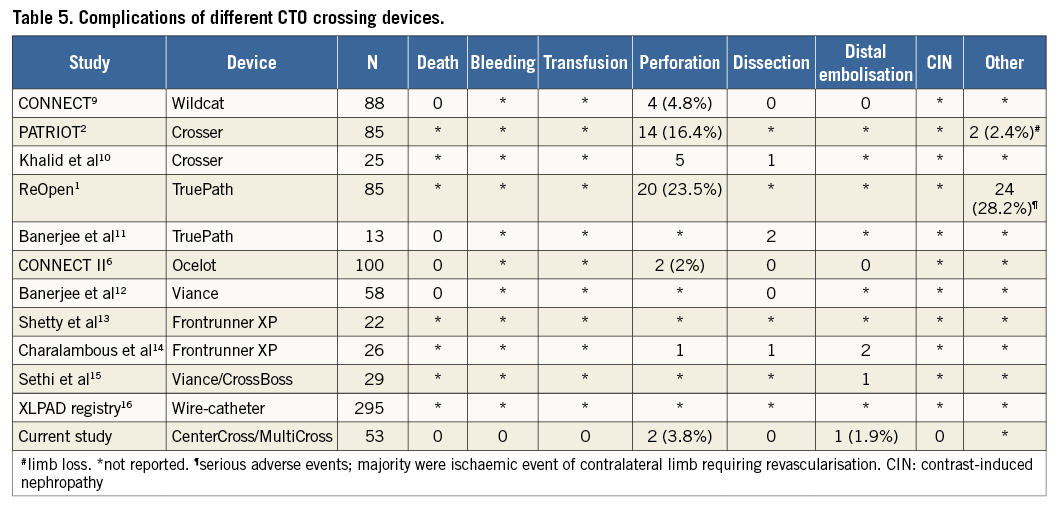
However, what can be stated is that the results of the current study are encouraging, demonstrating a relatively high procedural success rate of crossing lengthy lesions with a relatively short crossing time and contrast use with roughly comparable fluoroscopy times. Furthermore, it was encouraging that the current devices were safe and were associated with low complication rates, most of which were not life-threatening. Additionally, these favourable outcomes were achieved despite the fact that in our patients the vast majority of treated lesions were located in the popliteal artery and/or below this level (89%); this contrasts with most other studies where the majority of treated CTO were superficial femoral artery lesions1,2,6,9-14. Available data suggest that popliteal or infrapopliteal CTO are associated with significantly lower success rates, longer procedural times and higher complication rates as compared to suprapopliteal lesions15.
Our data suggested that CenterCross/MultiCross might have the potential to improve procedural success without compromising safety in patients undergoing peripheral CTO interventions. Additionally, unlike many other devices, CenterCross/MultiCross catheters are easy to use, eliminating the need for ultrasound or other ancillary guidance, minimising cost in these patients.
Limitations
Our study should be viewed in the light of its limitations. The major limitation was that our investigation was a non-randomised small case series without a concurrent control arm, particularly comparing it directly with other devices for crossing CTO. The sample size was small to modest given preliminary experience. This was a single-centre study with a single experienced operator. Therefore, it does not represent broad contemporary clinical experience. MultiCross and CenterCross are relatively new devices and, while there may be a learning curve associated with their use, we were unable to provide insight into this. We did not routinely grade the severity of peripheral arterial calcification, a characteristic that may have important implications for crossing as well as for the success of intervention in CTO. Thus, we are unable to provide any information on the implications of the severity of peripheral arterial calcification on the success rate of crossing with the devices used in our study and on the eventual procedural success. The effectiveness/efficacy and safety of these new devices in broader contemporary practice and in comparison with other devices as well as their cost-effectiveness in peripheral CTO interventions needs to be evaluated in future studies in a large cohort of patients undergoing PAD interventions.
Conclusions
In conclusion, the present study demonstrates that MultiCross and CenterCross devices were effective and safe for the recanalisation of peripheral CTO lesions that were not amenable to conventional guidewires. Further study is required to define the role of these novel devices in the treatment of complex lesions, particularly CTOs in patients with PAD.
| Impact on daily practice Treating a CTO lesion remains challenging because of difficulty in crossing the lesion. Attempts to cross are associated with significant radiation, contrast use and longer procedure time. CenterCross/MultiCross devices are novel tools to cross CTO lesions. Our data revealed that CenterCross/MultiCross devices were effective in crossing CTO lesions in a short time with a relatively safe profile compared to other commonly used CTO crossing devices. Our data showed that using these novel devices may improve patient safety, comfort and healthcare costs and expands the armamentarium for the treatment of CTO. |
Funding
The study was funded in part by modest grant support from Roxwood Medical, Inc., CA, USA.
The sponsors had no role in the study design, the analysis, the drafting and editing of the manuscript, the content of the final manuscript or the decision to publish these results.
Conflict of interest statement
T.P. Davis is on the advisory board for Roxwood Medical, Inc. The other authors have no conflicts of interest to declare.

