Abstract
The fast development of multislice computed tomography (MSCT) for the study of the coronary arteries makes foreseeable its application in many of the aspects related to repeated coronary interventions after stent implantation. This refers not only to the assessment of long term results of stenting and the identification of restenosis, but also the relationship of the prosthesis to coronary locations, such as bifurcations, coronary ostia, etc., which might be relevant in planning secondary revascularisation.
In this article, the current state of the art of MSCT in assessing coronary stents is reviewed, paying attention to current technical limitations, to the available evidence on its use in different settings and to future applications in the context of improved MSCT and stent technologies.
Introduction
Non-invasive coronary imaging by multislice computed tomography (MSCT) has rapidly developed over the past decade. The technique has become an accepted diagnostic option to rule out coronary artery disease in patients with stable anginal complaints. However, in patients who previously underwent percutaneous coronary intervention (PCI) CT angiography is challenged by metal-related high-attenuation artifacts, which compromise diagnostic accuracy.
CT stent imaging
Computed tomography creates cross sectional images by calculating the regional roentgen attenuation of the scanned object. An iodine containing contrast medium is injected to create a difference in attenuation between the blood and the surrounding tissues, which then allows for the detection of luminal obstruction. Conventional stents cause significantly more attenuation than natural tissues or contrast-enhanced blood because of the high atomic number of the metals. This strong roentgen attenuation results in artifacts, which interferes with the interpretation of the in-stent lumen on contrast-enhanced MSCT angiography.
The diameter of the stent struts (0.07-0.14 mm) is considerably smaller than the three-dimensional CT image elements (voxels), which optimally measure ≈ 0.4 mm in all directions. Because of the very high contribution in attenuation by the stents struts, the averaged attenuation value (in Hounsfield units) within the voxel will be affected proportionally, and increase. Additionally, the CT data is filtered during the image reconstruction process to decrease image noise. This “smoothing” results in increased attenuation values caused by the stent to extend beyond the limits of the voxel with the stent material. Further blurring around the stent may result from residual motion artifacts. The combined consequence is a so-called blooming effect, which increases the apparent stent strut size, and artificially narrows the lumen within the stent as a consequence. Even under stationary, in vitro conditions, most of the lumen within the stent is affected by the adjacent stent, and results in artificial lumen narrowing of up to 40% even with 64-slice CT technology1.
Another source of artifacts originates from the fact that the X-ray emitted from the roentgen tube is not monochromatic, but consists of a spectrum of frequencies with varying energy levels. The high-density material of the stent causes a disproportionate attenuation of low-energy photons, and a shift in the spectrum of the remaining X-rays towards a higher energy level. This results in an interpreted lower attenuation and a dark appearance adjacent to the metal. This shadowing, which can also be found around calcifications, may be falsely interpreted as soft tissue within the stented lumen.
The magnitude and consequences of these artifacts are variable and depend on the stent size and design, patient characteristics and scanning conditions2. Depending on the atomic number and atomic density, the attenuation characteristics of different metals vary. In comparison to stainless steel and cobalt stents, tantalum stents, or gold or platinum markers cause more attenuation. In an in vitro study of 86 different coronary stents, Maintz et al, demonstrated that the artifact-free in-stent lumen is approximately 50%, but varies between 3% and 73% for specific stent types3. Depending on the strut thickness and the strut density of the stent design artifacts will increase. Because the extent of the blooming is fairly constant, the relative effect on interpretation of the in-stent lumen decreases with larger stent diameter.
Assessability of stents on CT can be increased by optimising the acquisition and reconstruction protocol (Figure 1). Reconstruction of thin slices with sharper filtering will decrease the blooming effect and increase the visible lumen within the stent3. Increased levels of image noise, which occur when reconstructing thinner slices with sharper filtering, may necessitate higher tube currents during scanning. Stent artifacts combined with motion artifacts are likely to render the in-stent lumen non-interpretable. The temporal resolution of current CT technology varies between 80 and 200 ms, which is a considerable improvement compared to earlier technology, but still insufficient in patients with a fast heart rate. Heart rate modulation is advised in patients with higher heart rate who need to be examined by MSCT, particularly when the patient previously underwent stent implantation. Somewhat higher heart rates may be tolerated using dual-source CT instead of conventional single-source technology.
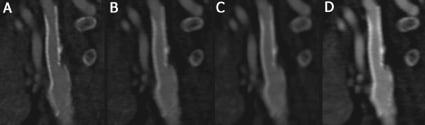
Figure 1. Dual-energy CT angiogram of a stent in a carotid artery reconstructed in four different ways: A) tube current 140 kV, slice thickness 0.6 mm, sharp kernel; B) 140 kV, 0.6 mm, softer kernel; C) 140 kV, thicker slices (1.0 mm), soft kernel; D) 80 kV, thin slices (0.6 mm), soft kernel.
Detection of in-stent restenosis by MSCT
Most patients who underwent percutaneous coronary intervention in the past will need diagnostic angiography again at some point in time. Recurrent symptoms may (or may not) be caused by in-stent restenosis or disease progression in non-treated coronary branches. A noninvasive alternative to catheter angiography is desirable. Employing consecutive CT technology numerous studies have been published that investigated the diagnostic performance of CT angiography after previous coronary stenting4-20. Insufficient image quality required exclusion of up to 46% of the imaged stents. Sensitivity for the detection of in-stent restenosis varied between 67% and 100%, with a specificity between 74% and 100%. The heterogeneous results of these studies can at least in part be explained by differences in stents type and size, the relatively small populations and the variable prevalence of disease. Several authors reported better image interpretability and diagnostic performance in stents with larger diameters and thinner struts4,5,8,12-14,16,19. Often stents smaller than 3.0 mm are considered unlikely to be well interpretable. Stents made of tantalum and those containing gold are less interpretable compared to stents made of stainless steel and cobalt alloys8. It has also been demonstrated that stent fracture can be imaged21, however, less than severe stent malapposition is beyond the reach of current CT technology.
In a meta-analysis of fourteen 16- and 64-slice CT studies by Vanhoenacker et al the pooled sensitivity to detect in-stent restenosis, after excluding 11% (95% CI: 4-20%) of the data, was 82% (72-89%), the specificity was 91% (83%-96%), pooled negative likelihood ratio and positive likelihood ratio were 0.20 (0.13-0.32) and 9.34 (4.68-18.62), respectively22. The authors could demonstrate that the exclusion rate was influenced by the number of detectors, stent diameter, strut thickness and the use of sharp filtering.
Including a number of recent 64-slice and dual-source CT studies, Table 1 suggests that the sensitivity of 64-slice CT has improved to ≥90%. While the prevalence of in-stent restenosis varies between 6 and 49%, the proportion of complete occlusion is often high (25-59%). Stent occlusion is easier recognised than in-stent stenosis (Figure 2). For those papers reporting separate results for occlusion and stenosis, pooled analysis shows that all occlusions are detected (sensitivity 100%), while only 82% of the 50-99% stenosis are identified6,7,10,12,14,15. Due to the blooming artifact, a minimal amount of neointimal hyperplasia is required for it to be recognised on CT. Van Mieghem et al showed with IVUS that the minimal amount of neo-intimal hyperplasia was at least 1 mm11, Manghat et al concluded from angiographic comparisons that <30% in-stent stenosis could not be well recognised on MSCT20. Although the diagnostic performance of current CT technology is improving, results are still considered insufficient for positive recommendations of unrestricted use of CT coronary angiography in patients with coronary stents23,24.
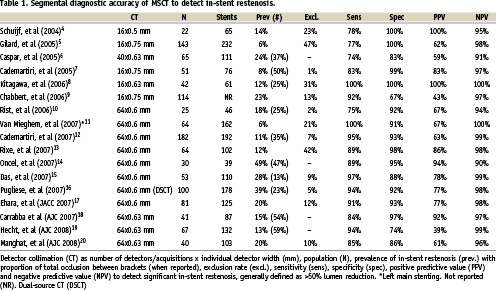
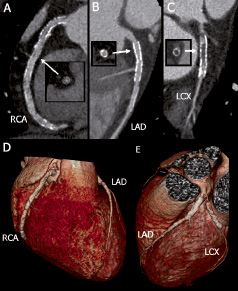
Figure 2. Three-vessel stenting with diffuse in-stent hyperplasia of an extensively treated right coronary artery (RCA, A and D), a fully patent left anterior descending coronary artery (LAD) with two stents (B, D, E), and an occluded stent in the left circumflex branch (LCX) with the distal lumen filled via collaterals (C and E). Note the preserved septal branch (panel B).
CT after left main stenting
Despite the known challenges, limited evidence suggests that severe obstruction of larger stents in the proximal coronary branches can be performed reliably using state-of-the-art CT technology. In this respect, a patient population of particular interest are those who underwent stenting of the left main coronary artery. Considering the challenges of noninvasive detection of left main in-stent restenosis by means of exercise testing or perfusion imaging, as well as the potentially disastrous consequences of unrecognised obstruction of the left main coronary artery, most patients will be subjected to invasive coronary angiography 3-6 months after the intervention. Because of the large diameter size of the left main coronary artery the in-stent lumen can be assessed well by MSCT in most cases (Figure 3). Van Mieghem et al, examined 74 patients after left main stenting using 16 and 64-slice CT, with stent diameters varying between 3.0 and 3.5 mm. In 70 patients, with sufficient image quality, all significant stenoses were detected, the specificity was 91%. Lower specificity was found in patient who underwent complex bifurcation stenting (80%), in comparison to patients after simple stenting (97%)11. This suggests that the use of CT can obviate the need for invasive angiography in the majority of patients. Furthermore, in those cases in which the stent was deployed in the ostium with partial protrusion of the prosthesis in the aorta, MSCT can avoid not only the difficulties of selective coronary catheterisation, but also the inherent risk of stent damage or deformation by the coronary catheter seen in such attempts. Finally, and following the considerations already made in a previous chapter about MSCT in surgical grafts in this same issue, the large diameter size and relative immobility of surgical grafts makes assessment of stents in these conduits well possible6 (Figure 4).
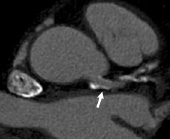
Figure 3. Assessment of a coronary stent implanted in the left main coronary artery. Despite significant coronary calcification, patency of the stent (arrow) can still be established.
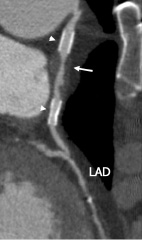
Figure 4. Saphenous vein graft from the aorta to the LAD. Two stents (arrow heads) are well interpretable, and apparently without significant in-stent restenosis. Between the both stents progressive graft disease can be observed (arrow).
Future development
CT innovations in terms of smaller detectors to reduce volume averaging and blooming, higher temporal resolution to reduce motion artifacts, dedicated filtering during image reconstruction, will continue to improve the interpretation of coronary stents. Improvements in stent design may also benefit non-invasive CT follow-up. Currently available stents with thinner struts show less blooming. Recently developed non-metallic, biodegradable stents are largely invisible on CT and allow reliable non-invasive follow-up by cardiac CT (Figure 5)25.
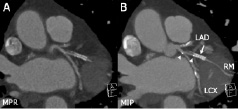
Figure 5. Marginal branch (RM) treated with a biodegradable, polylactic acid stent (BVS stent, Abbott Vascular, Redwood City, CA, USA), and overlapping conventional metal stent. The metal stent (arrow) causes extensive blooming, particularly on the maximum intensity projection image (MIP, B). The non-metal stent is invisible on CT except for the two radiopaque, platinum markers (arrow heads) at both ends of the stent. Mixed plaque on one side of the proximal marginal branch can still be appreciated. Multiplanar reformation (MPR).

