Introduction
In 2008, our group investigated the accuracy of quantitative measurements for lumen, vessel and plaque volume derived from multislice computed tomography (MSCT) scans in comparison with intravascular ultrasound (IVUS) greyscale in a cohort of 47 patients: an excellent correlation and a relatively acceptable agreement were shown1. More recently, Voros and colleagues reviewed the field and published a comprehensive analysis of the relationship between MSCT- and IVUS-derived measures for lumen and plaque area2. They concluded that for lumen and vessel areas good correlations exist with a coefficient higher than 0.7, and a systematic error of about 20%, MSCT values being consistently overestimated. Regarding percentage of atheroma volume, the correlation was only modest with a coefficient of correlation close to 0.5, and in general the Bland-Altman plot showed an acceptable mean difference though with relatively wide limits of agreement. The same authors also reviewed the capability of MSCT to characterise tissues, using the classical threshold of more than 150 Hounsfield units (HU) for calcified plaque, 30-149 HU for high-density non-calcified plaque, and -100 to 30 HU for low-density non-calcified plaque. Only a modest correlation was found for both calcified and non-calcified plaque area, and both components were excessively overestimated by CT angiography (CTA) with rather poor overall agreement. In the authors’ experience, high-density non-calcified plaque as quantified by MSCT had a closer, even if moderate, correlation with the percentage of fibrous tissue derived by IVUS virtual histology (IVUS-VH), whereas only a poor correlation existed between low-density non-calcified plaque and fibro-fatty tissue plus necrotic core. This component was apparently underestimated by CTA.
One of the intrinsic limitations of CTA in assessing plaque characteristics is the fact that the contrast density of the lumen impacts on the plaque density in the surroundings of the lumen. For instance, a plaque characterised as fibrous with 100 HU at the time of the peak contrast filling of the lumen (e.g., a density of 330 HU) could appear like a lipid plaque with 30 HU as soon as the contrast medium and its density are cleared out of the vessel lumen3,4 (Figure 1). Despite this limitation, vendors have created software algorithms which can differentiate between diverse types of tissue based on MSCT2,5-7. More recently, a fusion of near-infrared spectroscopy combined with IVUS and MSCT has provided in vivo three-dimensional (3D) distribution of lipid-core plaque in human coronary arteries8.
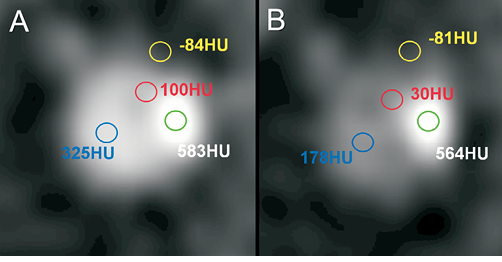
Figure 1. Impact of luminal contrast density on plaque density measurements in multislice computed tomography (adapted from reference 4). The same coronary artery cross-section is shown at the time of peak contrast filling (A) and in the delayed phase of contrast enhancement (B). Whereas the density (in Hounsfield units, HU) does not change for either coronary calcium (green circle) or the surrounding epicardial fat (yellow), plaque density (red) is significantly decreased, as soon as the contrast medium is cleared out of the vessel lumen (blue).
Beyond luminography and tissue characterisation, MSCT has so far been unable to provide a functional assessment of the severity of epicardial narrowings. Over the last 15 years, research in the field has made major progress and some landmark discoveries, such as the introduction and validation of patient-specific 3D blood flow analysis, the development of adaptive finite element models for simulating cardiovascular blood flow, the definition of physiologically realistic inflow and outflow boundary conditions as well as the coupled description of blood flow and vessel wall dynamics. Recently, methods for modelling coronary artery flow and auto-regulatory mechanisms have also been developed (Table 1).
Our group has actively participated in this line of research: in 2000, we published the true 3D reconstruction of coronary arteries in patients by fusion of angiography and IVUS, and quantitatively validated this method9. In parallel, the department was active in developing new methodological approaches for the assessment of coronary haemodynamics before and after coronary interventions, using intra-coronary pressure and flow velocity measurements, acquired with sensor-tip guidewires10.
Fractional flow reserve
Fractional flow reserve (FFR), the ratio of maximal blood flow on either side of a coronary artery stenosis (distal vessel flow divided by proximal vessel flow), was introduced almost two decades ago11, has been intensively validated and has demonstrated its clinical utility in deferring percutaneous treatment that would not have been appropriate in non-flow-limiting stenoses12. In clinical practice, beyond the temptation to treat unnecessarily (ad hoc on the table of the cathlab) a lesion that does not warrant percutaneous treatment, the (interventional) cardiologist is flooded with information derived from non-invasive angiography studies suggesting the presence of added coronary lesions next to the ones involved in the aetiology of an ischaemic event. In order to filter out the false positives (and taking into account the modest positive predictive value of CTA)13, a multitude of non-invasive tests are performed and further developed to define anatomy, to determine the functional repercussions of narrowing on the blood flow, or to assess the myocardial sequelae of ischaemia14-17. The tests involve different disciplines of investigation (radio-nuclear, echocardiography, magnetic resonance imaging), and constitute a severe financial burden for society18,19, and a substantial psychological nuisance for the patient submitted to this battery of diagnostic tests, sometimes redundant. The desire to have a single diagnostic modality providing all the information in one single non-invasive assessment is long awaited20.
Non-invasive FFR assessment with MSCT
FFRCT technology is based on three key underlying principles for the generation of physiologic models of coronary blood flow. According to the first principle, baseline coronary blood flow is met by myocardial demand for oxygen at rest. Allometric scaling laws of the form
Qcrest ![]() Mmyok
Mmyok
can be used to estimate physiological parameters: for instance, coronary flow (Qc) under baseline conditions given an organ mass Mmyo; k is the scaling factor21-23. Adherence to this principle enables calculation of total resting coronary blood flow relative to patient-specific left and right ventricular mass that can be quantified on the same MSCT scan. Naturally this assumption does not apply to patients with angina at rest and thus would prohibit analysis with FFRCT technology in them. The second principle claims that the resistance of the microcirculatory vascular bed at rest is inversely, but not linearly, proportional to the size of the feeding vessel as demonstrated in prior morphometry, shear stress autoregulation, and compensatory remodelling research24-30. In other words, healthy and diseased blood vessels adapt to the amount of flow they carry. Power law relationships of the form
rpk=rd1k+rd2k
apply to the coronary arteries; autoregulation of wall shear stress provides the mechanism to explain observed power laws inherent in flow-diameter relationships26,27. The third principle says that the coronary microcirculation has a predictable response to adenosine, which is produced as soon as the heart lacks oxygen, with a breakdown of ATP, resulting in a release of adenosine. Exogenous administration of adenosine elicits the maximum hyperaemic responses by producing complete relaxation of the smooth muscle cells lining the resistance arterioles. Coronary flow reserve (ratio of flow during maximum hyperaemia to resting flow) is modulated by both epicardial and microcirculatory resistance31. This is based upon a prior landmark study by Wilson and colleagues, who identified a predictable response of the total coronary resistance to hyperaemia induction by intravenous or intracoronary administration of adenosine. What is most important with FFRCT technology is that it does not require any modifications in coronary CTA acquisition protocols, additional imaging, or administration of additional medicine.
Cardiovascular computational fluid dynamics
Any application of computational fluid dynamics to structures as small as the coronary circulation bed requires unique capabilities for finite element mesh generation and modification32. A flow of an incompressible fluid, such as blood, submitted to a high driving pressure (e.g., 100 mmHg) could be highly accelerated when a small additional gradient of pressure is applied: this could generate significant changes in wall shear stress which require customised solving tools. Within current algorithms, finite element meshes can iteratively adapt their resolution in an anisotropic fashion, while the distribution of element size and density can be modified according to computation requirements33,34. Whereas in areas of less complex flow the mesh elements may be coarser and less dense, structured layers of elements are required near the vessel wall (boundary layers) in order to enhance the accuracy of wall shear stress computations, especially in exceedingly curved vessels35. On the other hand, flow-pressure variations in the coronary arteries cannot be directly measured as they are inherently part of the solution. Coronary flow distribution is modulated by both the resistance of the downstream vascular bed and the intra-myocardial pressure due to ventricular myocardium contraction36. Flow is not always proportional to pressure: thus, their relationship at the outlets of the coronaries is better expressed in the form of outflow boundary conditions coupling the 3D epicardial domain to the microvasculature. Conversely, the influence of preload, heart rate, contractility and cardiac output can be modelled in appropriately set inflow boundary conditions37.
Based upon the aforementioned three principles, a dedicated algorithm using cardiovascular computational flow dynamics was created (HeartFlow™; HeartFlow Inc., Redwood City, CA, USA) for non-invasive FFR derivation. The anatomy of the coronary arterial bed of a particular patient is not known a priori and has to be extracted from a patient-specific medical imaging dataset. MSCT can provide an accurate coronary geometric model, including branching and pathology specific to a patient. Based upon this geometric information, a volumetric finite element mesh with anisotropic refinement and boundary layers is generated in order to compute numerical results (Figure 2). Using a proprietary algorithm the heart-vessel interaction can be defined, whereas time-varying coronary resistance for each coronary branch can be determined relative to intra-myocardial pressure and microvasculature impedance. This latter component can be represented by a so-called lumped (zero-dimensional) parameter model, which resembles an electric circuit, including resistive and capacitive elements (Figure 3)36. Finally the complex fluid properties of the blood are entered into the model, in order to refine the computations.
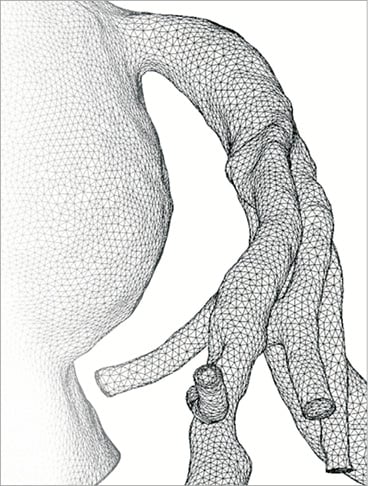
Figure 2. Finite element model of a left coronary artery. The distribution of element size and density can be iteratively adapted to computation requirements.
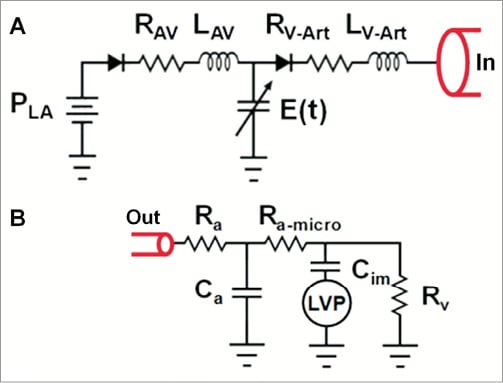
Figure 3. Lumped (zero-dimensional) parameter models used in cardiovascular blood flow simulations. A) Lumped parameter heart model coupling the left heart side to the aortic inlet (In) of a closed loop system comprising the systemic and pulmonary circulation. The left heart side model consists of left atrial pressure PLA, mitral valve, atrioventricular valvular resistance RAV, atrioventricular inductance LAV, aortic valve, ventriculo-arterial valvular resistance RV-Art, ventriculo-arterial inductance LV-Art, and left ventricular (LV) pressure. The LV pressure is modelled with time-varying LV elastance E(t). B) Lumped parameter coronary vascular bed model coupled to a coronary branch outlet (Out). This model consists of coronary arterial and venous resistance (Ra and Rv, respectively), coronary arterial microcirculation resistance Ra-micro, coronary arterial capacitance Ca, intramyocardial vascular capacitance Cim, and LV pressure; LV pressure stands for intra-myocardial pressure for branches perfusing the left ventricle and septum.
Upon completion of the blood flow analysis, mean coronary pressure is extracted from the computer analysis performed under maximum hyperaemic conditions. The FFRCT is defined as the computed mean coronary pressure distal to a lesion divided by the computed mean blood pressure in the aorta under conditions of simulated maximum hyperaemia.
The included moving image (Moving image 1) represents an actual measurement of FFRCT provided by applying the basic scientific principles, as integrated in the proprietary algorithm briefly and succinctly described above. The customer has to upload the patient case onto a dedicated website: the very first step is segmentation of the major vessels, followed by plaque detection and removal, providing smooth luminal surfaces of the vascular bed. The model is trimmed and outflow boundaries are identified, and a patient-specific volumetric finite element mesh is generated and solved. Coronary blood flow and pressure are subsequently computed under conditions of maximal hyperaemia. On the moving image file, one can observe the luminogram of a left coronary artery as derived from the MSCT scan: particles flowing through the blood stream help visualise the vascular laminar flow proximal to a bifurcation (lesion) and the region of high velocity (in red on Moving image 1) with flow reversal and oscillatory shear stress immediately distal to it. In the left upper part of the movie, the pressure gradient is depicted whereas the FFRCT value is continuously tracked on the coronary circulation, while scrolling from the distal vessel toward the main stem, where the FFRCT gets normalised. The algorithm provides the option to perform virtual percutaneous coronary intervention (PCI) of the diseased segment (by, for instance, virtually implanting a stent), and simulate the functional result of the treatment by re-calculating the FFRCT values. Then the physician can choose from among a number of alternative treatment strategies and implement the one which would yield the best result.
The diagnostic performance of FFRCT and coronary CTA-derived diameter stenosis (50% cut-off) compared to invasive FFR used as a reference standard has recently been reported in 159 vessels (103 patients)38; an FFRCT ≤0.80 was considered diagnostic of lesion-specific ischaemia. Measurements for FFRCT and FFR were highly correlated (coefficient=0.72, p<0.001), whereas FFRCT slightly underestimated invasive FFR (mean difference 0.02±0.12, p=0.02). Accuracy, sensitivity, specificity, positive predictive value and negative predictive value of the FFRCT and coronary CTA-derived diameter stenosis >50% are shown in Figure 4.
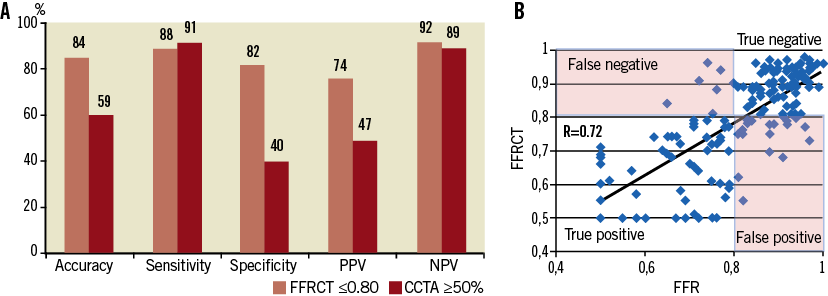
Figure 4. Diagnostic performance of FFRCT compared to invasive FFR in 159 vessels38. A) Accuracy, sensitivity, specificity, positive and negative predictive value (PPV and NPV, respectively) for FFRCT and CCTA-derived diameter stenosis (50% cut-off). B) Correlation of FFRCT with invasive FFR values was high (R=0.72, p<0.001). A cut-off of ≤0.80 was considered diagnostic of lesion-specific ischaemia for both FFRCT and FFR. CCTA: coronary CT angiography; FFR: fractional flow reserve
Over the past decade, non-invasive imaging using MSCT has lived up to its promises: luminography and tissue characterisation have made major progress in quantification, due to continuous increase in spatial (16, 64, 128, 256 and 320 detectors/slices) and temporal resolution, despite some inherent limitations of x-ray regarding tissue characterisation. The adjunction of functional assessment for flow-limiting lesions is a quantum leap in the non-invasive evaluation of patients prior to PCI with the option of virtual treatment planning. Consequently, a single MSCT study will ultimately provide us with comprehensive “one-stop” non-invasive evaluation of the coronaries.
Examples of clinical cases
Case 1
The first clinical example concerns a patient who five years ago received a bioresorbable everolimus-eluting scaffold in the proximal right coronary artery (RCA). At 6-month and 24-month follow-up, the conventional angiography did not show any significant stenosis in the scaffolded segment. At five-year follow-up, the MSCT scan showed a patent, non-stenotic scaffolded area demarcated by the two radiopaque platinum markers (black arrows, Figure 5). On the maximum intensity projection, two small calcified plaques are visible distal to the scaffold. Non-invasive functional assessment showed that all three vessels exhibited distally FFRCT values above a threshold of 0.80: specifically, there was no gradient in FFRCT along the scaffolded segment.
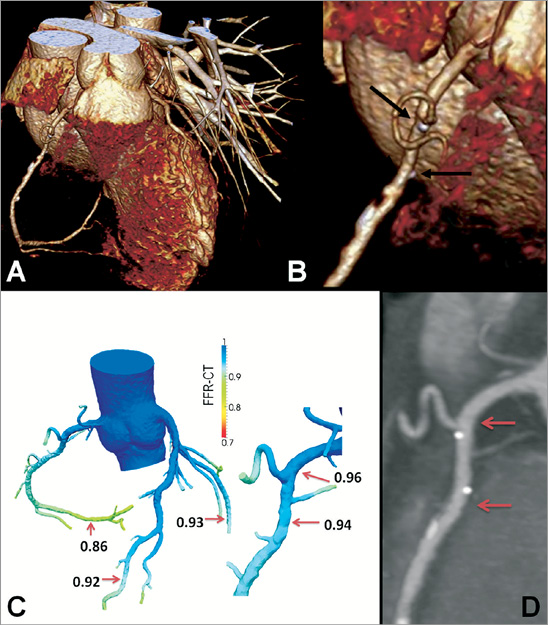
Figure 5. Use of non-invasive FFR for follow-up of a bioresorbable scaffold. A) Three-dimensional volume-rendered CT angiogram of a right coronary artery. Magnification of the proximal artery segment (B) shows the platinum markers of the resorbed scaffold (black arrows). C) Non-invasive FFR derivation did not show ischaemia in any coronary artery. Specifically, FFRCT measured 0.96 and 0.94 at the inflow and outflow of the scaffolded segment (red arrows on the curved multi-planar CT image) (D). The colour scale of the CCTA-derived computer models is based on the FFRCT values. CCTA: coronary CT angiography; FFR: fractional flow reserve
Case 2
The second case concerns a patient with obstructive disease in the proximal left anterior descending (LAD) coronary artery, causing ischaemia in both LAD and the first diagonal branch (D1). Disease extends from the proximal LAD to the take-off of the first septal branch. Using the computer models based on non-invasive FFR derivation (Figure 6), one could describe this anatomy as a Medina (1,0,0) lesion at the LAD-D1 bifurcation plus one separate distal LAD lesion just proximal to the take-off of the septal branch. Alternatively, one could describe two sequential (1,0,0) bifurcation lesions, if the septal is considered to be a major vessel and therefore warrants protection. Following these diagnostic considerations, the treatment plan could be shaped accordingly. In the latter diagnostic approach, one could consider a provisional T-stent procedure for the diagonal branch with guidewire protection for the septal branch. On the other hand, if the septal is perceived to be a small vessel, one could opt for a culotte approach using a Tryton Side-Branch Stent™ (Tryton Medical, Inc., Newton, MA, USA), provided that the distance between the ostium of the circumflex and the ostium of the first diagonal is compatible with the length of the proximal part of the Tryton stent; subsequently, a long drug-eluting stent would be placed in the LAD, possibly covering the ostium of the septal branch.
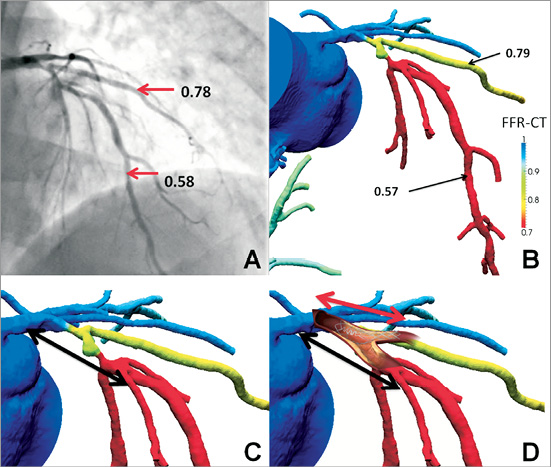
Figure 6. Use of non-invasive FFR in procedural strategy for bifurcation PCI. Proximal LAD disease causes ischaemia in both the distal LAD and first diagonal branch (D1); invasive (A) and non-invasive estimates (B) are concordant. The colour coding of the computer models facilitates ischaemia localisation. Dependent on diagnostic interpretation (including the size of the first septal branch), either a provisional T-stent (C) or a Culotte approach using a Tryton Side-Branch Stent™ (D) can be followed. The black arrows indicate the position where the drug-eluting stent will be placed in the proximal LAD. FFR: fractional flow reserve; LAD: left anterior descending coronary artery
Case 3
The last case concerns a 65-year-old male patient with stable angina: his coronary risk factors were hypertension and hypercholesterolaemia. MSCT angiography disclosed two stenotic lesions, one in the ostium of the LAD and one in the mid portion of the RCA, respectively (not shown). The mid to distal LAD had a FFRCT of 0.71 vs. 0.74 with the invasive approach (Figure 7). Left main stem (LM) anatomy could be interpreted as either a trifurcation with one vessel segment diseased (LAD ostium), hence a Medina (0,1,0,0) lesion or as a trifurcation with two diseased vessel segments (also involving the LM), therefore a (1,1,0,0) lesion. Irrespective of diagnostic interpretation, one would consider stenting the mid intermediate branch lesion without touching the trifurcation. Nevertheless, according to interpretation one could consider treating the LAD ostium alone, or alternatively place a stent from the LM into the proximal LAD with a provisional 2-3 stent strategy. Virtual PCI of the LAD ostium alone resulted in a limited improvement, FFRCT slightly increasing from 0.77 to 0.80. The most striking observation was a drop in FFRCT along the LM measuring 0.81 in its mid portion. Virtual treatment of LM alone already normalised FFRCT in the mid LAD (FFRCT=0.90). The combined treatment of LM and LAD ostium resulted in further improvement of the FFRCT up to a value of 0.93.
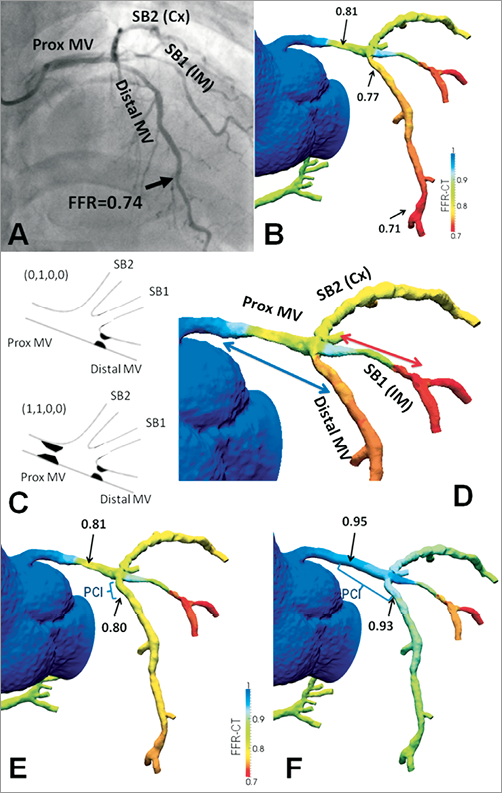
Figure 7. The yield of virtual treatment planning with non-invasive FFR measurements in an ambiguous left main (LM) stem anatomy. Both invasive (A) and non-invasive FFR estimates (B) showed ischaemia in the mid-distal LAD. According to diagnostic interpretation (see case description) (C) one can opt for treating the LAD ostium alone or place a stent from the LM into the proximal LAD (blue arrow) (D). The former option does not fully alleviate ischaemia (E), whereas the latter one does (F). Cx: (left) circumflex coronary artery; FFR: fractional flow reserve; IM: intermediate (branch); LAD: left anterior descending coronary artery; MV: main vessel; PCI: percutaneous coronary intervention; SB: side branch
Acknowledgements
The authors would like to acknowledge the contribution of Charles A. Taylor, PhD and John H. Stevens (HeartFlow Inc., Redwood City, CA, USA) regarding the description of HeartFlow™ technology and the analysis of clinical case examples as well as Bon Kwon Koo (Seoul National University Hospital, Seoul, South Korea) and James K. Min (Cedars-Sinai Heart Institute, Los Angeles, CA, USA) for providing cases 2 and 3 from the DISCOVER-FLOW population.
Glossary
allometry: change in proportion of various parts of an object/organism as a consequence of growth
anisotropy: the property of being directionally dependent. An example of an anisotropic material is wood, which is easier to split along its grain than against it
impedance: frequency analogue of resistance
lumped (model): zero-dimensional model
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Example of FFRCT measurements including virtual treatment.

