Abstract
Transradial (TR) coronary intervention is associated with fewer access-site-related bleeding complications and is independently associated with a lower risk of mortality following PCI compared to procedures undertaken through the femoral route. However, recent studies that have undertaken imaging of the radial artery through the use of IVUS and OCT, as well as histological studies, suggest that TR cardiac catheterisation is associated with significant injury to the radial artery wall resulting in significant endothelial cell dysfunction. The vascular endothelium plays a central role in the regulation of vascular tone, angiogenesis and vascular remodelling through the release of vasoactive mediators in response to a variety of stimuli. Hence, trauma to the vascular endothelium and subsequent changes in endothelial cell function may contribute to patterns of injury such as intimal hyperplasia and radial artery occlusion observed following TR cardiac catheterisation. Such injury patterns to the radial artery following TR procedures may limit the success and future utility of the TR approach. Minimisation of radial artery injury should be a key procedural component of procedures undertaken through the transradial approach.
Introduction
Since the introduction of the transradial (TR) approach for diagnostic coronary angiography in 1989 by Campeau1 and percutaneous coronary intervention (PCI) in 1993 by Kiemeneij2, the radial artery has been increasingly adopted as the primary access site for cardiac catheterisation. Recent advances in the miniaturisation of devices has further facilitated the TR approach as a routine technique and expanded the complexity of the procedures which can be performed through this route3. Reported benefits of the TR approach include fewer access-site-related bleeding complications3-6 and reduction in the risk of mortality following PCI7,8. Although post-procedural early and late radial artery occlusion rates have historically been thought to be low9-11, more recent data suggest that rates of non-occlusive radial artery injury may be significant, which may potentially limit the use of the transradial route in future PCI procedures12. Therefore, for the transradial approach to remain a feasible access-site option in patients undergoing future repeat PCI procedures, the mechanisms that underlie radial artery injury need to be better understood with procedural steps taken to reduce the potential for such injury.
The purpose of this review is to provide an overview of recent studies which suggest that both occlusive and non-occlusive radial injury following TR cardiac catheterisation is significant and to discuss potential pathophysiological mechanisms that contribute to these processes. Finally, we discuss procedural and pharmacological strategies which minimise radial injury during TR cardiac catheterisation. Patterns of injury are summarised in Figure 1 and detailed below.
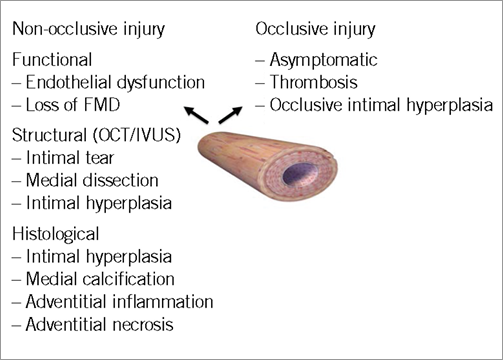
Figure 1. Patterns of radial artery injury documented following transradial cardiac catheterisation.
Non-occlusive injury
FUNCTIONAL CHANGES IN THE RADIAL ARTERY FOLLOWING TR CARDIAC CATHETERISATION
The endothelium is visible as a monolayer with flattened nuclei lining the inner vessel wall, and is the largest organ in the body. In an adult it is composed of 1-6×1013 cells, and covers a surface area of approximately 7,000 m2 13. The endothelium plays a central role in the regulation of vascular tone, angiogenesis and vascular remodelling through the release of vasoactive mediators in response to a variety of stimuli14. Endothelial dysfunction is a hallmark of vascular diseases and is often regarded as a key early event in the development of atherosclerosis15. Recently, work has focused on the effects of transradial cardiac catheterisation on flow-mediated dilatation (FMD) as a surrogate of radial artery endothelial dysfunction. Flow-mediated dilatation (FMD) is an in vivo bioassay of NO-mediated endothelial function16 in which an increase in blood flow within a blood vessel mediates a vasodilatory response through the release of nitric oxide (NO) from the vascular endothelium15. Burstein et al17 recently demonstrated that FMD was reduced by 70% immediately post 6 Fr TR PCI and was virtually abolished at nine-week follow-up. Similarly, Heiss et al18 showed a decrease in FMD by 50% six hours post procedure using a 5 Fr sheath, although FMD returned to baseline in non-smokers at 24 hours. Others using 6 Fr PCI systems have also demonstrated that FMD was similarly reduced, although it returned close to baseline levels at 12 weeks19. Interestingly, this and other studies18 also demonstrate inhibition of the FMD response distal to the sheath insertion site, suggesting that the guide catheter itself may induce trauma to the vascular endothelium independently from that occurring following introducer sheath insertion.
Functional changes observed following TR cardiac catheterisation are not only isolated to the endothelial-dependent functions of the radial artery but may also influence vascular smooth muscle function. Both Burstein17 and Dawson19 have shown impaired vasodilatory response of the radial artery to glyceryl trinitrate (GTN) following transradial catheterisation with full19 or partial17 recovery at longer time points, implying a decreased responsiveness of the smooth muscle cells within the radial artery wall to the effects of NO. These data suggest that utilisation of the transradial approach is associated with significant radial endothelial and vascular smooth muscle cell dysfunction which may persist in the longer term.
Structural changes in the radial artery following TRA
ACUTE INJURY
Radial artery optical coherence tomography (OCT) performed in patients who have undergone 6 Fr TR cardiac catheterisation has revealed significant rates of vascular trauma acutely20. Both intimal tears and medial dissections within the radial artery have been observed, with rates as high as 43.8% and 23.3%, respectively, in the distal vessel. Even proximally, where only the guide catheter would have contact with the radial artery lumen, rates of intimal tears of 17.8% and medial dissections of 20.5% have been reported. Both intimal tears and medial dissections were greatest in frequency close to the point of introducer sheath insertion, and lowest in frequency in the proximal radial artery where only the guide catheter (with a smaller outer diameter than the corresponding introducer sheath used) would have contact. This suggests a relationship between radial artery luminal diameter and introducer sheath/guide outer diameter for acute injury. The outer diameter of a 6 Fr introducer sheath (2.48 mm) is similar to the reported inner diameter of the distal radial artery21,22, which may account for the high prevalence of radial injury observed in the OCT study of Yonetsu20. Interestingly, a high prevalence of radial artery trauma in the proximal radial artery where no sheath was present was also observed where radial artery luminal diameter (3.5 mm) was significantly greater than the outer diameter of a 6 Fr guide catheter (typically 2.0-2.1 mm). This may point towards mechanisms mediated by guide-catheter-induced trauma which occurs through radial artery spasm, resulting in the catheter coming into contact with and traumatising the radial artery luminal wall, irrespective of radial artery luminal diameter. Whilst it is possible that the exchange wire may also contribute to such trauma, this is less likely as a mechanism, due to the less traumatic flexible j-tipped wire edge, compared to the larger diameter and stiffer guide catheter, unless the radial artery spasm is severe and resistance to wire passage is encountered, where wire-induced trauma would be more important.
CHRONIC INJURY
Several OCT and IVUS studies examining radial arteries in patients undergoing repeat transradial cardiac catheterisation have documented evidence of chronic injury to the radial artery. In the OCT study of Yonetsu20, the authors documented significant intimal hyperplasia in the radial arteries of patients undergoing repeat TR procedures with smaller radial artery luminal areas compared to patients who underwent a first TR procedure. Interestingly, this intimal hyperplasia extended to areas distal to the introducer sheath position, indicating that the guide catheter itself can induce a chronic injury response in the vessel. Similar findings have been reported in studies which have performed IVUS examination of radial arteries in patients who have undergone repeat TR procedures21. Histological analysis of radial arteries harvested to serve as conduits in patients undergoing CABG revealed significant intimal hyperplasia, medial calcification, adventitial inflammation and necrosis in patients who had undergone previous TR cardiac catheterisation procedures, whereas these histological patterns of injury were absent in non-catheterised arteries23. Similar histological patterns of injury (although less prevalent) were documented in the proximal radial artery, suggesting that radial trauma is limited not only to the sections of radial artery in contact with the introducer sheath but also those in contact with the guide catheter.
OCCLUSIVE INJURY
Whilst radial artery occlusive injury is less common than the non-occlusive injury described above, it is often asymptomatic, since the dual blood supply to the hand provides collateral protection against hand ischaemia, although this has rarely been reported in isolated case reports after transradial interventions24. Furthermore, there is growing evidence that a third artery, the interosseous artery, may also contribute to the blood supply of the hand and therefore provide further protection against hand ischaemia25,26. Radial occlusion is important, since it has significant implications for repeated catheterisation procedures and availability of arterial conduits for surgical revascularisation or haemodialysis fistulae.
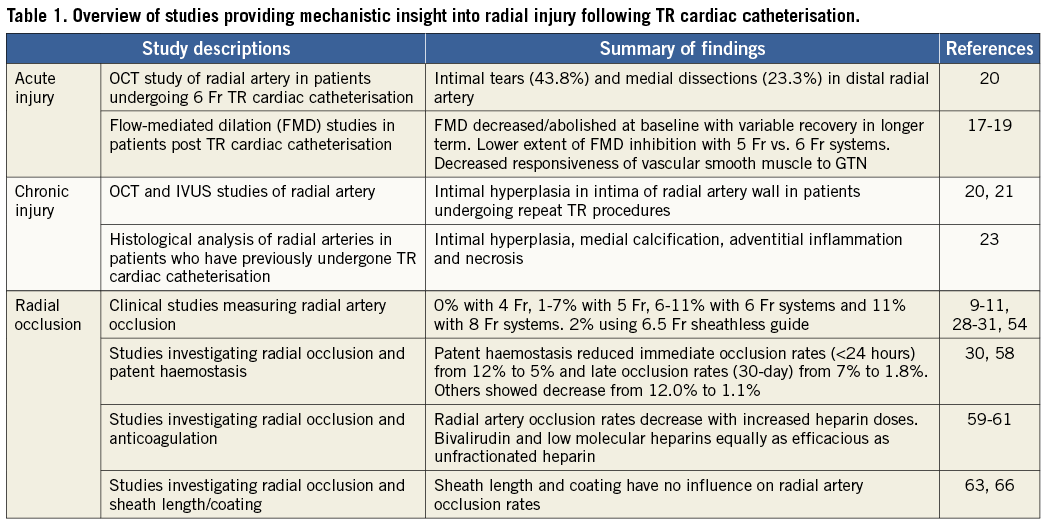
Radial artery occlusion rates relate to the size of the guide catheter used, more specifically the ratio between the outer diameter (OD) of the introducer sheath used and the inner luminal diameter (ID) of the radial artery, so that a ratio of greater than 1 (OD introducer sheath > ID radial artery) predicts radial artery occlusion27. In patients who underwent TR PCI in whom ultrasound measurement of the inner diameter of the radial artery was performed, the OD of the introducer sheath was > ID of the radial artery in 15%/29% of males and 28%/60% of females when using a 6 Fr/7 Fr introducer sheath, respectively27. Radial occlusion rates increase with the size of introducer sheath used: for example, radial occlusion rates of 0% have been reported with 4 Fr systems28 and 1-7% with 5 Fr systems10,29,30, 6-11% with 6 Fr systems9-11 and 11% with 8 Fr systems31. It is likely that radial occlusion rates are significantly greater than those detected clinically by the absence of a radial pulse, since the occluded radial artery distal stump has been found to have up to 70% of mean arterial pressure because of macro-collateral circulation from the palmar arches32 leading to a palpable pulse. For example, in one study following 6 Fr transradial catheterisation radial occlusion rates defined by the absence of a radial pulse were documented at 4.4%, whereas the absence of radial artery flow measured was 10.5%11. Radial occlusion has been reported to occur as frequently as 18% in some series using 6 Fr systems immediately post TR catheterisation, although at seven-day follow-up rates of 10.5% radial occlusion have been reported11. Similarly, the PROPHET study using 5 Fr systems has reported occlusion rates of 12% at 24 hours post procedure, although this declined to 7% at 28 days30, suggesting that there is spontaneous recanalisation of occluded radial arteries periprocedurally and that radial occlusion rates are significantly greater than previously appreciated in the immediate post-procedural period. The presence of radial artery anomalies may also contribute to an increased risk of radial artery occlusion. Hence, an appreciation of the radial anomalies that may potentially be encountered during transradial cardiac catheterisation is important. Table 1 provides a summary of studies providing insight into the mechanisms of radial artery injury following TR cardiac catheterisation.
Relevance
Whilst the relevance of radial artery occlusion is evident, the chronic radial artery injury patterns described above, such as intimal hyperplasia, are also important since they may limit future transradial approaches. In a study of 812 patients undergoing transradial cardiac catheterisation, Sakai et al12 have shown that procedural success declines with subsequent procedures, so that a third TRA procedure was only possible in 90% and 80% of men and women, respectively, whilst a fifth TRA procedure was only possible in 70% and 50% of men and women, respectively, due to narrowing or occlusion of the radial artery. Whilst PCI procedures in this series were performed with 5 or 6 Fr introducer sheaths, the ability to undertake successful consecutive procedures which require larger diameter guide catheters (7 Fr) for greater back-up support or delivery of 7 Fr compatible equipment would be even more limited.
Mechanisms
Imaging modalities such as OCT and IVUS as well as histological and functional studies have demonstrated significant structural and functional changes to the radial artery post transradial catheterisation. It is easy to imagine the potential for significant disruption to the endothelial lining of the radial artery in cases where the size of the radial sheath exceeds the inner diameter of the radial artery. Such trauma to the endothelium accounts for the disruption of FMD that has been observed following transradial cardiac catheterisation, but may also play a key role in the development of chronic patterns of injury such as intimal hyperplasia and radial occlusion.
Intimal hyperplasia is an inflammatory and proliferative process that occurs within the radial artery as a result of injury to the vessel wall and endothelium following sheath insertion and guide catheter manipulation within the radial artery. The process of intimal hyperplasia is the earliest response to endothelial injury and consists primarily of proliferation and migration of medial smooth muscle cells into the intima33 and deposition of extracellular matrix. The vascular endothelium regulates cellular adhesion to the arterial wall and the local production of growth-regulatory molecules34 which play a key role in the intimal hyperplastic response following vessel wall injury. At the molecular level, the events occurring during intimal hyperplasia are initiated and maintained by transcription factors. Activation of transcription factors can result in the altered expression of pathophysiologically relevant genes regulating smooth muscle cell hypertrophy, migration and deposition of extracellular matrix35. One such transcription factor, nuclear factor kB (NF-kB), plays a critical role in the pathophysiological processes leading to the development of neointimal hyperplasia36, blocking NF-kB activation by the NF-kB decoy oligodeoxynucleotides (ODN) which attenuates neointimal formation after balloon injury in animal models37. Whilst specific inhibitors of NF-kB activation have not been studied to determine whether they inhibit intimal hyperplasia post TR cardiac catheterisation, trimetazidine (TMZ) has recently been shown to be protective against the loss of FMD post transradial cardiac catheterisation38 potentially through inhibition of NF-kB activation as outlined below.
The development of radial artery occlusion post TR cardiac catheterisation is thought to occur through two main mechanisms: through occlusive intimal hyperplasia and, more commonly, through the development of thrombus. The concept of endothelial injury in the development of thrombosis has been present for over 40 years39. Trauma to the endothelium either during transradial catheterisation or post-procedurally during attempted haemostasis may result in endothelial cell trauma with exposure of thrombogenic connective tissue, leading to a predisposition to thrombus formation and subsequent radial artery occlusion. Increased expression of procoagulatory molecules such as von Willebrand factor (vWF) and tissue factor (TF), enhanced thrombin generation, platelet aggregation and adhesion, and fibrin deposition shown to occur following vascular endothelial trauma further favour thrombus formation. Occlusive “plugs” retrieved from occluded radial arteries following transradial cardiac catheterisation demonstrate organised thrombus, supporting the view that thrombosis is a key process that underlies the early radial artery occlusion observed40. Minimisation of endothelial cell trauma and inhibition of thrombus formation are key components in reducing radial occlusion following TR PCI.
Minimising radial injury
Minimising radial injury should be a key procedural component of TR PCI and consists of endothelial protection, minimisation of endothelial cell/vessel wall trauma and minimisation of thrombus formation (Figure 2).
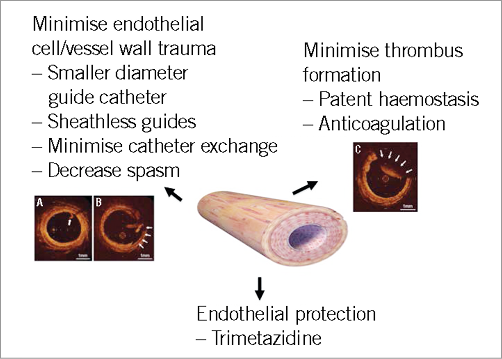
Figure 2. Summary of methods to minimise radial injury during/following transradial cardiac catheterisation. Panels A, B and C are OCT images (derived from reference 20) of radial arteries which illustrate an intimal tear (A; arrows), medial dissection (B; arrows) and thrombosis (C; arrows).
Modulation of endothelial function
Trimetazidine (TMZ) decreases the utilisation of free fatty acids for energy production by inhibiting mitochondrial 3-ketoacyl CoA thiolase (3-KAT) activity in beta-oxidation, thus shifting metabolism towards glucose41. In addition to metabolic effects, TMZ was previously shown to have non-metabolic benefits: inhibition of inflammatory response, improvement of endothelial dysfunction, and reduction of oxidative stress42,43. In a randomised controlled study of 60 patients undergoing 5 Fr TR coronary angiography, TMZ was given at a dose of 20 mg tds post coronary angiography for a period of 10 weeks, and endothelial cell function, measured as FMD, was assessed at both baseline and at 10-week follow-up38. Post-procedurally, a significant reduction in the FMD response was observed which was similar in the control and TMZ groups, although by 10 weeks FMD was restored in the TMZ group. Whilst the authors of this study did not investigate the mechanisms through which TMZ improved FMD, studies performed in heart failure patients have documented improvements in endothelial function mediated through a reduction in lipid hydroperoxides, formed through oxidation of lipids by reactive oxygen species42. Other studies have also shown that TMZ inhibits NADPH oxidase activation and the production of reactive oxygen species44. NADPH oxidase activation with the production of reactive oxygen species is known to activate NF-kB45, which is thought to play a central role in intimal hyperplasia in the vessel wall following vascular wall injury as outlined above. Inhibition of NADPH oxidase activation by TMZ with subsequent inhibition of NF-kB activation remains an attractive mechanism through which TMZ may potentially inhibit intimal hyperplasia.
Smaller guide catheters
Use of smaller diameter guide catheters may reduce endothelial and radial wall injury and so minimise radial occlusion rates. Radial occlusion rates increase with increasing size of guide catheter systems used with rates of 0% having been reported with 4 Fr systems28, 1-7% with 5 Fr systems10,29,30, 6-11% with 6 Fr systems9-11 and 11% with 8 Fr systems31. However, direct evidence demonstrating that radial artery occlusion rates are reduced using smaller guide catheters is less clear. In a randomised trial of 171 patients undergoing TR PCI with 5 vs. 6 Fr guide catheters, radial occlusion rates were less in the 5 Fr cohort compared to the 6 Fr cohort (1.1% and 5.9%, respectively)10, although this was not statistically significant. Other studies have shown similar radial occlusion and procedural success rates when comparing transradial PCI route 5 or 6 Fr guide catheters46,47. Many of these studies have been statistically underpowered to detect small differences in radial occlusion rates. Furthermore, radial occlusion is a relatively infrequent manifestation of radial injury and studies have not been performed comparing 5 Fr and 6 Fr guide catheters on more commonly observed sequelae of radial injury, such as abnormalities in FMD reflecting endothelial dysfunction, intimal hyperplasia or acute structural injuries such as medial dissection and intimal tears. Indirect evidence suggests that non-occlusive radial injury may be less significant using smaller guide catheters. Sanmartin48 demonstrated that 4 Fr sheaths do not decrease FMD at 24 hours whereas Heiss et al18 demonstrated a 50% reduction in FMD at six hours post procedure with normalisation of the response within 24 hours using 5 Fr guide catheters. In studies using 6 Fr guide catheters, FMD either remained abolished at follow-up17 or only recovered over longer follow-up times19.
Although procedural success rates between 5 Fr and 6 Fr guide catheters have been shown to be similar in simple PCI cases, use of 5 Fr guide catheters or smaller may limit the complexity of lesions tackled. For example, back-up support of guide catheters has been shown to be a function of their size49: hence, in complex cases where increased back-up support is necessary, a 5 Fr guide is inadequate. Furthermore, treatment of bifurcation lesions with two stent/balloon strategies, cases necessitating rotational atherectomy such as heavily calcified lesions, or cases that require adjunctive devices such as thrombectomy devices and distal protection devices50, or stent delivery catheters51,52, will necessitate 6 Fr or larger guide catheters.
The outer diameter of radial introducer sheaths is typically 2 Fr sizes larger than the outer diameter of the guide catheter. Therefore, a potential means by which guide catheter size can be maintained with minimisation of radial trauma is to perform sheathless transradial PCI. We have previously described the use of the Asahi sheathless guide catheter system53,54 (ASAHI Intecc, Aichi, Japan), which is available in 6.5 Fr and 7.5 Fr sizes. The 7.5 Fr version has an external diameter of 2.49 mm which is significantly less than the external diameter of a conventional 6 Fr introducer sheath (2.62 mm), whilst the outer diameter of a 6.5 Fr sheathless guide (2.16 mm) is equivalent to a 4.5 Fr conventional sheath. In a prospective study performed in 100 consecutive patients undergoing transradial PCI using 6.5 Fr sheathless guide catheters we demonstrated procedural success rates of 100% with no procedural complications recorded associated with the use of the catheter54. Radial occlusion rates were 2%, comparing favourably with occlusion rates reported with conventional 6 Fr systems (6-11%)9-11. More recently, other groups have modified conventional guide catheters for use without an introducer sheath and reported procedural success using virtual 2 Fr55 and 3 Fr56 guide catheters. Whilst the absence of an introducer sheath may limit radial injury, it may still occur, mediated by the guide catheter itself. For example, abnormalities in FMD have been demonstrated in the brachial artery following transradial PCI18, with evidence of intimal tears, medial dissections and intimal hyperplasia demonstrated on OCT in radial arteries following transradial PCI in areas significantly distal to introducer sheath placement20. Furthermore, the absence of an introducer sheath may paradoxically increase radial trauma, particularly during guide catheter exchanges. Hence, the role of sheathless guide catheters in minimising radial injury remains unclear.
Patent haemostasis and anticoagulation
Different devices have been developed to achieve local compression of the radial artery post transradial cardiac catheterisation with comparable haemostatic efficacy57. These devices function through the application of localised pressure over the radial artery puncture site to ensure haemostasis. However, application of localised pressure to the radial artery may result in endothelial cell trauma with exposure of thrombogenic connective tissue, which, along with prolonged stasis of blood, predisposes to thrombus formation and subsequent radial artery occlusion. In one prospective study of 275 patients undergoing TR cardiac catheterisation, absent radial flow was present in 62% of cases following application of haemostatic bandages, and was still absent in 18% of cases following removal of bandages11. Indeed, in this study an occlusive compression technique was associated with a significantly higher rate of radial occlusion at follow-up11. These observations have led to the hypothesis that interruption of the process of thrombus formation at the time of haemostasis, by maintaining radial artery flow at the site of puncture, so-called patent haemostasis, may decrease subsequent radial artery occlusion. In the PROPHET study, in which patients were randomised to patent haemostasis or conventional pressure application for haemostasis, immediate occlusion rates (<24 hours) were decreased from 12% to 5% and late occlusion rates (30-day) were decreased from 7% to 1.8% in the patent haemostasis arm30. Similar prospective studies such as the RACOMAP study performed with pneumatic compression devices in which compression was guided by mean arterial pressure (hence maintaining flow within the radial artery during haemostasis) have also revealed decreased radial occlusion rates, 12.0% vs. 1.1% in favour of the patent haemostasis arm58.
Another means of reducing the formation of thrombus within the radial artery post-procedurally is through the use of anticoagulation. Use of unfractionated heparin has been shown to decrease radial occlusion rates. In one study, asymptomatic radial artery occlusion was noted in 71% of patients undergoing TR cardiac catheterisation in the absence of heparin use, decreasing to 24% in those patients receiving 2,000-3,000 units of heparin, and 4.3% in patients receiving 5,000 units of heparin59. Other studies have shown both low molecular heparins60 and bivalirudin61 are as equally efficacious as unfractionated heparin in reducing radial occlusion post transradial procedures. Finally, more recent data have suggested that, in cases of radial artery occlusion, transient homolateral ulnar artery compression can result in recanalisation of a significant proportion of occluded radial arteries in patients treated with low-dose heparin62.
Prevention of spasm
Whilst it is well documented that radial artery occlusion rates increase significantly once the outer diameter (OD) of the introducer sheath is greater than the inner luminal diameter (ID) of the radial artery27, the inner luminal diameter of the radial artery can become markedly decreased through the development of radial spasm. The occurrence of radial spasm may potentially increase endothelial cell and vascular trauma within the radial artery. Radial spasm occurs commonly in TR procedures, with rates reported as high as 18-22% in some studies63,64. Radial spasm can be reduced through the use of hydrophilic-coated introducer sheaths65,66 as well as the use of pharmacological agents such as verapamil and nitroglycerine64,67,68.
It has been hypothesised that reduction of radial spasm may result in a reduction in radial occlusion rates. For example, studies which have investigated the utility of hydrophilic-coated introducer sheaths on the development of spasm have demonstrated that, although radial spasm rates were significantly reduced when using the hydrophilic-coated introducer sheath (4% vs.18%), no differences were observed in radial occlusion rates (3.5% vs. 3.5%)63. In a separate 2×2 factorial study of 790 consecutive patients in which the influence of introducer sheath length and hydrophilic coating on radial spasm was studied66, a reduction in radial spasm rates was associated with the use of a hydrophilic sheath, although this had no effect on radial occlusion observed. Whilst it appears that reducing radial spasm through the use of hydrophilic sheaths has no influence on radial occlusion rates, there are no studies that have influenced the effects of targeting radial spasm on structural trauma to the radial artery such as intimal tears and medial dissections and the subsequent development of intimal hyperplasia. A recent proof of concept study has evaluated the protective effect of NO-coated introducer sheaths in juvenile porcine femoral arteries of a similar size to human radial arteries and has shown that these sheaths were associated with a significantly higher vessel diameter at both the access site and proximal and distal to it with significantly reduced luminal thrombosis periprocedurally69. Furthermore, at one-week follow-up, lower intimal inflammation score, less luminal thrombosis, and reduced intimal hyperplasia were observed in NO-sheath arteries at the injury site. Future studies may focus on novel pharmacotherapy-coated sheaths which target the molecular pathways that contribute to intimal hyperplasia analogous to drug-eluting stents and restenosis.
Conclusion
Transradial coronary intervention is associated with fewer access-site-related bleeding complications and is independently associated with a lower risk of mortality following PCI. Recent data suggest that transradial cardiac catheterisation is associated with both structural and histopathological injury patterns to the wall of the radial artery, resulting in significant endothelial cell dysfunction. Trauma to the vascular endothelium and subsequent changes in endothelial cell function may contribute to patterns of injury such as radial artery occlusion and intimal hyperplasia that are known to limit the success of future transradial procedures.
Minimisation of radial artery injury and occlusion should be a key procedural component of all transradial procedures. Radial injury and occlusion relate to the size of the introducer sheath/guide catheter used in comparison to the inner luminal diameter of the radial artery. Therefore, radial injury may be minimised through the use of smaller diameter guide catheters or through the use of sheathless guide catheters, minimisation of catheter exchanges and minimisation of radial spasm periprocedurally, either through pharmacological means or through the use of coated sheaths/guide catheters. Minimisation of thrombus formation within the radial artery post transradial catheterisation procedures will minimise radial occlusion. Hence, routine anticoagulation with heparin periprocedurally and patent haemostasis post-procedurally are recommended. More recent novel pharmacotherapies, such as trimetazidine, target the endothelium and may represent a novel approach to protection against radial injury. Radial occlusion is a relatively infrequent manifestation of radial injury and future studies of novel pharmacotherapy/transradial equipment should focus on more commonly observed sequelae of radial injury, such as abnormalities in FMD reflecting endothelial dysfunction, acute structural injuries such as medial dissection and intimal tears with the ensuing future development of intimal hyperplasia. Minimising radial artery injury should be considered a key component of all transradial procedures.
| Impact on daily practice The radial artery has become the predominant PCI access site due to associated reductions in mortality observed in high-risk populations mediated through reductions in access-site-related bleeding complications. Injuries to the radial artery such as intimal hyperplasia or radial occlusion limit the success of future procedures. Radial injury may be minimised through the use of smaller diameter guide catheters, minimisation of catheter exchanges and minimisation of radial spasm either through pharmacological means or through the use of coated sheaths/guide catheters. Minimisation of thrombus formation within the radial artery post transradial catheterisation procedures will minimise radial occlusion, hence routine anticoagulation with heparin periprocedurally and patent haemostasis post-procedurally are recommended. Minimising radial artery injury should be considered a key component of all transradial procedures. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

