Abstract
Aims: Endothelium dysfunction has been reported in patients (pts) undergoing transradial catheterisation. Alterations of the hand microcirculation possibly associated with systemic inflammation have never previously been reported. We aimed at investigating possible alteration of hand endothelial microcirculation secondary to transradial heart catheterisation.
Methods and results: We randomised 40 pts with stable angina undergoing coronary angiography to either transradial (TR, n=20) or transfemoral (TF, n=20) approach. At baseline (BL), 24 hours (24 hrs) and 30 days (FU: follow-up) after catheterisation we assessed: a) peripheral endothelial function (EndoScore [ES]) by peripheral arterial tonometry (EndoPAT); b) biomarkers of endothelial turnover (sE-Selectin) and inflammation (hs-CRP). No clinical or angiographic differences were observed between the two groups. At 24 hours, ES (BL: 0.42±0.27 vs. 24 hours: 0.27±0.19, p<0.05) significantly decreased in the TR group, but not in the TF group (BL: 0.44±0.34 vs. 24 hours: 0.45±0.39, n.s.). Both sE-Selectin and hs-CRP increased significantly at 24 hours in all pts. At 30 days, we observed in the TR group a restoration of ES (FU: 0.44±0.34, n.s. vs. BL; p<0.05 vs. 24 hours), while no difference was observed in the TF group. A reduction towards baseline was observed in both groups of sE-Selectin and hs-CRP.
Conclusions: A transient impairment of the digital microcirculatory endothelial function is observed in patients undergoing transradial diagnostic catheterisation, beyond local mechanical injury at the arterial access and on top of the systemic inflammatory response associated with heart catheterisation.
Abbreviations
BL: baseline
∆ES : absolute reduction of EndoScore
EndoPAT: peripheral arterial tonometry
ES: EndoScore
FU: follow-up
hsCRP: high-sensitivity C-reactive protein
IV: intravenous
pts: patients
PWA: pulse wave amplitude
sE-Selectin: soluble E-Selectin
TF: transfemoral
TR: transradial
Introduction
Endothelium dysfunction has been reported in patients undergoing transradial catheterisation, at the level both of the radial and of the brachial artery1. This impairment was limited to the catheterised arm, and it was mainly attributed to the mechanical manipulation of the arterial conduit, excluding any possible dysfunction of the downstream microcirculation, i.e., due to embolisation or inflammatory locoregional alterations. In addition, significant histological changes of the radial artery have been observed limited to the puncture site: i.e., intimal hyperplasia, medial inflammation, and tissue necrosis2. However, these studies neither performed a direct assessment of the microcirculation downstream from the cannulated radial artery, nor definitely excluded possible systemic influence to the observed local endothelium. On the one hand, in fact, the occurrence of a distal microcirculatory dysfunction during transradial catheterisation might partially explain the impaired hyperaemic radial artery response. On the other hand, diagnostic heart catheterisation, which by itself is associated with systemic inflammation in patients with stable coronary artery disease3, might contribute to a generalised impairment of the peripheral vascular endothelium4.
In our study we hypothesised that the endothelial dysfunction observed in patients undergoing transradial catheterisation is also associated with alterations to the downstream microcirculation, beyond possible systemic inflammation and mechanical endothelial damage induced by the catheter manipulation.
Methods
STUDY DESIGN
A total of 40 patients with stable angina undergoing elective coronary angiography were consecutively and prospectively assessed for peripheral endothelial function. Exclusion criteria were: a) acute coronary syndrome; b) chronic inflammatory disease (i.e., coeliac disease, vasculitis, lupus, chronic obstructive pulmonary disease, irritable bowel disease, arthritis, and psoriasis); c) clinically active malignancy or end-stage renal failure; d) previous coronary bypass graft surgery; e) previous radial cannulation; f) abnormal Allen’s test result consistent with insufficient ulnar collateral supply; g) Raynaud’s syndrome.
The study protocol is shown in Figure 1. Vasoactive medications were withheld at least 24 hours before the endothelial function assessment, as previously described5,6. All measurements and venous blood sampling were performed in the morning, after 12 hours fasting status, from the right arm (through an 18-G cannula in the cephalic vein), before coronary angiography (baseline [BL]), 24 hours after (24 hrs) and 30 days after coronary angiography (follow-up [FU]).
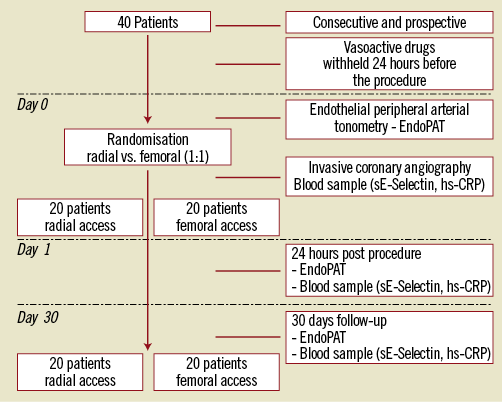
Figure 1. Study design.
Patients were randomised (1:1) to either transradial (TR) or transfemoral (TF) access. Assignment to one of the two groups was determined by a computer-based randomisation system, and randomisation assignment for each patient was kept in a sealed envelope.
Changes in the ongoing medical therapy were not allowed during the study period. Moreover, for patients in whom revascularisation was indicated (n=5 in TR group; n=4 in TF group), follow-up was planned not earlier than 30 days after the angiography and in any case before the planned revascularisation procedure. The local ethics committee approved the study protocol, and all patients provided written informed consent.
HEART CATHETERISATION
In patients undergoing transradial catheterisation, the right radial artery was cannulated with a 6 Fr, 10 cm-long sheath (Radifocus®; Terumo Europe NV, Leuven, Belgium). After sheath insertion, all patients received 0.2 mg nitroglycerine intra-arterially to prevent vasospasm and 2500 IU of unfractionated heparin IV. Cordis Multipack catheters (Cordis, Johnson & Johnson, Amersfoort, The Netherlands) were used for left heart catheterisation. If necessary, additional catheters were also used. At the end of the procedure, the sheath was removed immediately, and a wrist clamp (TR band 18 ml; Terumo Europe NV, Leuven, Belgium) was applied for 4 hrs (15 ml). In patients undergoing transfemoral approach, the right femoral artery was cannulated with a 6 Fr, 11 cm-long sheath (Avanti®+; Cordis, J&J, Roden, The Netherlands). After sheath insertion, 2500 IU of unfractionated heparin IV was given. Irrespective of the allocation, all patients received an IC bolus of 0.2 mg nitroglycerine.
ENDOTHELIAL FUNCTION ASSESSMENT
Peripheral endothelial function was measured by digital pulse amplitude using endothelial peripheral arterial tonometry (Endo-PAT2000; Itamar Medical, Caesarea, Israel), as previously described7,8. The EndoPAT detects plethysmographic pressure changes in the finger tips caused by the arterial pulse and translates this into a peripheral arterial tone (PAT). With the hyperaemic response observed after the occlusion of the brachial artery, the changes in EndoScore reflect endothelium-mediated changes in vascular tone, which have been shown to depend on nitric oxide synthesis9,10. A peripheral arterial tonometry finger probe was placed at the tip of each index finger, and a blood pressure cuff was placed on the study arm corresponding to the same arm used for the radial access (Figure 2). After a five-minute resting period (baseline), the blood pressure cuff was inflated up to 20 mmHg above the systolic pressure in order to achieve complete arterial occlusion for five minutes. Next, the blood pressure cuff was deflated, and the peripheral arterial tonometry recording was extended for an additional five minutes (hyperaemia). The endothelial response was assessed by using the Framingham reactive hyperaemia index (EndoScore [ES])8, according to the following formula:
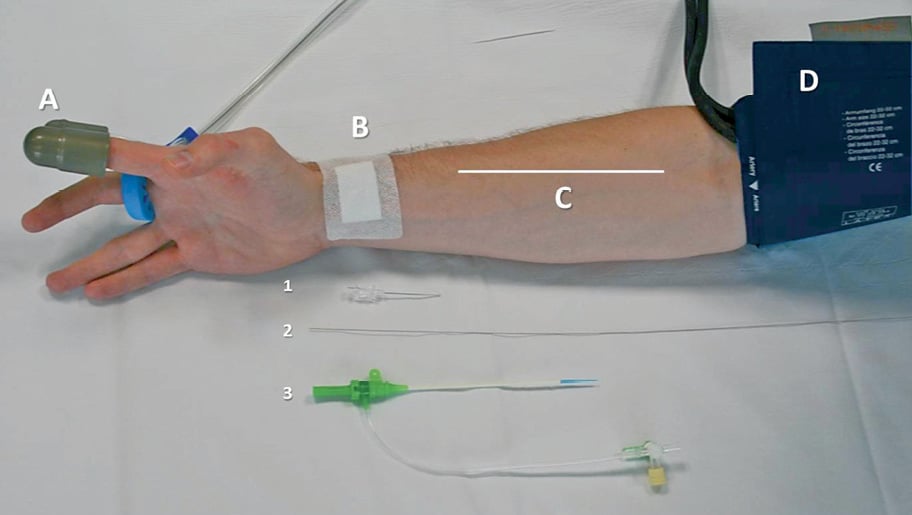
Figure 2. Schematic representation of the experimental protocol. Peripheral endothelial function was measured by digital arterial tonometry at the tip of the finger (A), after reactive hyperaemia was induced by sphygmomanometer inflation (D). The right arm was the study arm in all patients, while the left arm served as control arm. All patients undergoing transradial catheterisation were punctured on the right radial artery (B) that was retrogradely cannulated for sheath insertion (1-3). This model made it possible to dissect out mechanical endothelial denudation possibly occurring through the manipulation of the radial artery (C) after the passage of sheaths and catheters from an endothelial dysfunction induced by the release of inflammatory agents either locoregionally or systemically.
EndoScore (ES)=ln
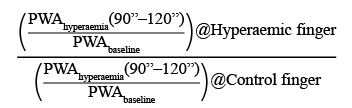
ln is the natural base log and PWA is the mean pulse wave amplitude.
The extent of endothelial impairment possibly occurring in the two groups was assessed by the absolute reduction of ES (∆ES) obtained as follows: ES at 24 hrs – ES at baseline.
ES was also assessed, on the right arm, in an additional control group of six stable angina patients undergoing transfemoral coronary angiography, at BL and 24 hrs after the heart catheterisation, in order to evaluate the possibility that transradial wrist clamp might have affected the endothelial function assessment. These patients fulfilled the inclusion-exclusion criteria mentioned above and vasoactive medications were withheld 24 hours before the protocol. A wrist clamp was applied on the right wrist for four hours after the transfemoral procedure.
BIOMARKERS ASSESSMENT
Plasma sE-Selectin was assessed as a marker of endothelial activation and damage by using the enzyme-linked immunosorbent assay (ELISA) method with a commercially available kit (R&D Systems, Abingdon, UK). The kit has a minimum detectable dose of 0.009 ng/ml and an intra- and inter-assay variation of less than 10%. Serum high-sensitivity CRP (hs-CRP) was assessed as a marker of systemic inflammation with the BDII System (Siemens AG, Munich, Germany) using laser nephelometry. The CardioPhase® hs-CRP method (Siemens) is an in vitro diagnostic test to measure C-reactive protein levels in human serum (mg/ml) quantitatively, with an inter-run assay variation of less than 5%.
Statistical analysis
We based our sample size calculation on a previous paper demonstrating a near 50% impairment in endothelial function after transradial catheterisation at the level of the arterial segment exposed to potential mechanical damage and catheter manipulation (i.e., the radial artery upstream from the puncture site and the brachial artery)1. In particular, we hypothesised that a similar degree of endothelial impairment will also occur downstream from the radial puncture site (i.e., digital circulation, not involved in the catheter manipulation), as compared with no significant changes in patients undergoing transfemoral catheterisation. The EndoScore in patients undergoing transfemoral approach was estimated at 0.47 with a standard deviation (SD) of 0.1911,12. We calculated that 16 to 20 patients per group should be included to detect a difference of 35 to 40% in EndoScore between patients undergoing transradial and patients undergoing transfemoral catheterisation (alpha: 0.05, statistical power: 0.80)13.
Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables are presented as mean±standard deviation or median with interquartile range as appropriate. Categorical variables are reported as frequencies and percentages. Normal distribution was tested with the D’Agostino-Pearson omnibus K2 test. Changes in EndoScore and sE-Selectin within groups were compared using one-way ANOVA for repeated measurements with Newman-Keuls post hoc test. Changes in hs-CRP were compared using the Friedman test with Dunn’s post hoc test.
Comparisons between continuous variables were evaluated using an unpaired t-test. Comparisons of hs-CRP between the TR and TF groups at the three respective time points were performed with the Mann-Whitney test. Comparisons between categorical variables were evaluated using the two-tailed Fisher’s exact test. A p-value of <0.05 was considered statistically significant.
Results
STUDY POPULATION
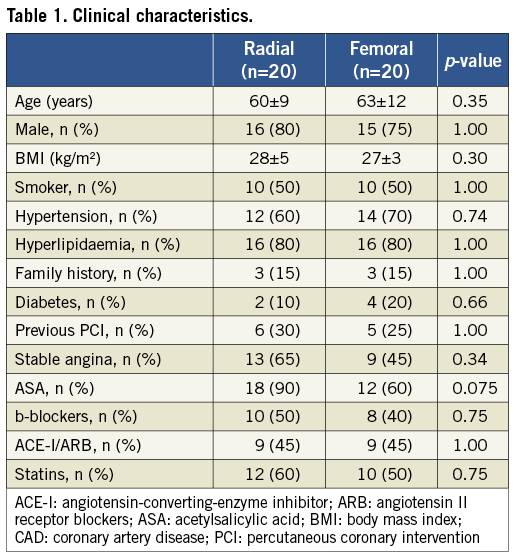
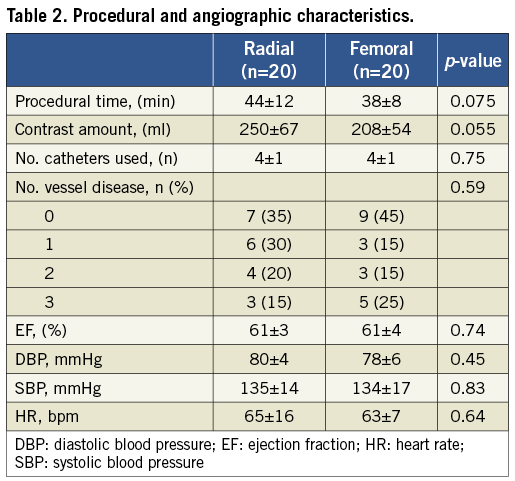
Clinical characteristics of the patients are reported in Table 1. There was no significant clinical difference between the two study groups. Likewise, no significant differences in procedural and angiographic features were observed, with the exception of a trend towards longer procedure time and higher contrast volume in the TR group (Table 2). All patients had a successful invasive diagnostic procedure, with no access site-related complications. Experimental measurements at baseline, 24 hours and 30 days were obtained in all patients.
PERIPHERAL ENDOTHELIAL FUNCTION
Changes in peripheral endothelial function during the study period are shown in Table 3 and Figure 3. In the TR group, PWAbaseline did not change significantly at the three study points (p=0.37). The ES decreased significantly at 24 hrs (p<0.05 vs. baseline), returning to baseline levels at 30 days (p<0.05 vs. 24 hrs; n.s. vs. baseline). In the TF group, PWAbaseline did not change significantly at the three study points (p=0.67). A significant change in ES was observed neither at 24 hrs nor at 30 days. The endothelial impairment was more pronounced in the TR group as compared with the TF group (∆ES: -0.16±0.25 vs. 0.01±0.20, p=0.022).
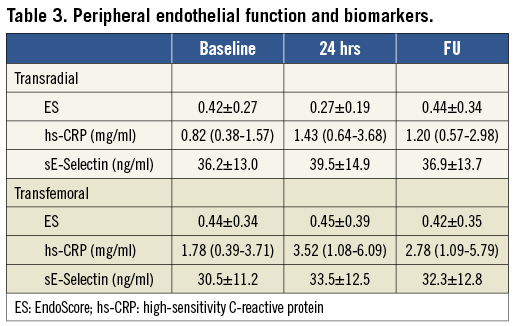
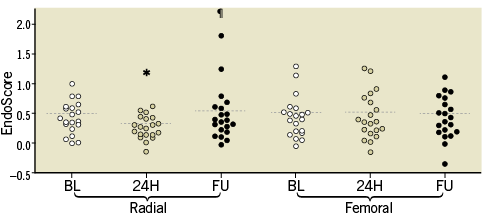
Figure 3. Changes in peripheral endothelial function. *p=0.008 vs. BL; ¶p=0.036 vs. 24 hours.
In the control group of six stable angina patients no differences were found in ES between the two study time points (BL: 0.49±0.10 vs. 24 hrs: 0.48±0.12, p=0.38), suggesting that wrist clamp does not per se affect the microvascular endothelial function.
BIOMARKERS
Changes in biomarkers are shown in Table 3. In the TR group, hs-CRP increased significantly at 24 hours (p<0.05 vs. baseline), with no further change at 30 days (n.s. vs. baseline and 24 hours). In the TF group, hs-CRP increased significantly at 24 hours (p<0.05 vs. baseline), with no further change at 30 days (p<0.05 vs. baseline; n.s. vs. 24 hours). Of note, hs-CRP was not different between the TR and TF groups at the three respective time points (data not shown).
In the TR group, only a trend towards a sE-Selectin increase was observed at 24 hours. In the TF group, sE-Selectin increased at 24 hrs (p<0.05 vs. baseline), with no further change at 30 days (p<0.05 vs. baseline; n.s. vs. 24 hours). Of note, sE-Selectin was not different between the TR and TF groups at the three respective time points (data not shown).
Discussion
In the present randomised study, we observed an impairment of digital microcirculatory endothelial function in patients undergoing transradial diagnostic catheterisation. This alteration was completely restored at 30 days, and occurred beyond the local mechanical injury at the arterial access and on top of the systemic inflammatory response associated with heart catheterisation.
Endothelial denudation and significant histological changes of the radial artery have been demonstrated after transradial catheterisation proximal to the puncture site2. Moreover, transradial catheterisation has also been associated with endothelial dysfunction of both the radial and the brachial artery1. This was linked to the mechanical vascular damage produced by the insertion of the introducer sheath and by the passage of catheters during the catheterisation. Alternatively, this endothelial impairment could be attributed to the systemic inflammatory response which frequently occurs during heart catheterisation, as a consequence of the contrast injection and possible aortic or coronary vessel wall injury induced by the manipulation of guidewires and catheters3,4.
Our findings confirm and further extend the available evidence. In our patients, endothelial function was assessed by EndoPAT at the tip of the finger in the study arm (right) and in the contralateral arm (left) that served as internal control. This model enabled us to explore: a) in patients randomised to the transradial approach, possible endothelial impairment occurring at digital microcirculation, downstream from the puncture site, therefore not only at the level of the radial artery subjected to the mechanical trauma associated with retrograde cannulation and manipulation; b) in patients randomised to the transfemoral approach, possible endothelial impairment linked to the systemic inflammation potentially occurring during the catheterisation, therefore unrelated to the local tissue injury induced by the arterial puncture (remote from the site where endothelial measurement is performed). Using this model, we demonstrated for the first time a significant post-procedural ipsilateral impairment of the digital microcirculatory endothelial function in patients undergoing transradial catheterisation.
In these patients, we also observed an increase in sE-Selectin and hs-CRP, suggesting a systemic activation of the endothelial turnover as well as an inflammatory response.
However, digital endothelial function was completely restored at 30 days. Despite this, biomarkers were still found to be elevated, supporting the concept of local endothelial impairment beyond the systemic alterations. Importantly, this could not be solely attributed to the mechanical endothelial denudation or vasospasm of the radial artery, as previously reported1.
Our findings extend these previous observations by demonstrating a transient endothelial dysfunction downstream from the damaged vascular segment, probably due to a locoregional release of inflammatory mediators. In addition, the lack of changes of baseline pulse wave amplitude along the three study points excludes a distal microembolisation as an alternative mechanism, in agreement with previous findings14,15. This concept is also confirmed by the lack of changes in digital endothelial function in patients undergoing transfemoral catheterisation, despite a comparable biomarker response.
If the EndoPAT had also been technically feasible at the level of the toes (therefore downstream from the femoral puncture site), we might also have been able to detect this transient endothelial alteration in patients undergoing transfemoral catheterisation.
Recently the EndoPAT evaluation has been challenged as a tool for reliable assessment of changes in endothelial function occurring during acute interventions, i.e., after smoking and hyperglycaemia16. However, these findings were not confirmed by other studies. In patients with type 2 diabetes mellitus under continuous glucose monitoring, fluctuations of blood glucose were significantly and independently correlated with changes in EndoScore17. In obese patients with metabolic syndrome, changes in endothelial function after an oral fat load were comparable for both EndoPAT and brachial ultrasound flow-mediated dilation18. An acute pharmacologic inhibition of von Willebrand factor has been associated with a significant EndoScore increase19. These controversial results might be due to the different study design and to the heterogeneity of the patient population investigated. With elderly patients, for example, the time course of the hyperaemic response is different from young subjects, i.e., the time frame to reach maximal vasodilatation after brachial artery occlusion is significantly prolonged20. In the automated analysis, the EndoPAT software uses a fixed time frame during the hyperaemic response to calculate the EndoScore. This potential confounder should be acknowledged, though the randomisation process adopted in our study has limited its impact.
Our findings have the following clinical implications. 1) Digital endothelial impairment during transradial heart catheterisation, even though transient, might potentially trigger hand ischaemia injury in patients already at risk for hand vasospasm as in cases of scleroderma or Raynaud’s disease14. In these patients the transfemoral approach should be preferred. 2) Use of digital pulse tonometry in assessing endothelial function is ever increasing not only for clinical investigations7,8,11,12 but also in daily clinical practice21-25. This method still remains valid in patients undergoing transradial catheterisation, though clinicians and investigators should be aware of the transient alterations of the digital endothelial function occurring post procedure.
Limitations
Even though the sample size was calculated and the power was adequate to detect possible differences in the primary outcome, we acknowledge that this remains a small pilot study. A number of other uncountable potential confounders might have played a role in the observed differences. For rigorous minimisation of potential bias, on the one hand we chose a randomised design for our study, while on the other hand we limited all possible external interventions, i.e., patients who underwent ad hoc percutaneous coronary intervention were excluded, to avoid confounders due to the revascularisation and to the change in medical therapy usually occurring thereafter.
Furthermore, few patients underwent FFR measurements at the time of the basal angiography (four in the transradial group and five in the transfemoral group). Here, hyperaemia was induced with intravenous infusion of adenosine (140 μg·kg−1·min−1). Although we cannot exclude an effect of adenosine metabolites on peripheral endothelial function, this is limited (if at all) considering that: a) patients undergoing adenosine infusion were equally distributed between the two groups; b) adenosine has a very short half-life (5-30 seconds)26; therefore, it is unlikely still to detect adenosine-induced endothelial activation at endothelial assessment performed the day after the procedure.
In addition, we did not perform any test to assess the media response, although changes in EndoScore of the study finger (subject to the hyperaemic response) are corrected with changes of the contralateral finger, thus accounting for potential autonomic influence at the level of media.
Finally, we do not have direct demonstration of the local release of biomarkers potentially contributing to the digital endothelial impairment. However, it should be taken into account that: a) the local release of biomarkers would have been masked by the systemic inflammatory reaction and endothelial activation related to the diagnostic procedure; b) we were able to detect an endothelial microvascular impairment despite the above background inflammatory response in the transradial group but not in the transfemoral group.
Conclusions
A significant impairment of the digital microcirculatory endothelial function occurs in patients undergoing transradial diagnostic catheterisation. This alteration was completely restored at 30 days, and occurred beyond the local mechanical injury at the arterial access and on top of the systemic inflammatory response associated with heart catheterisation.
Funding
This work was supported by an unrestricted grant from Meijer Lavino Foundation for Cardiovascular Research. L. Di Serafino was supported by a grant from the Italian Society of Cardiology.
Conflict of interest statement
The authors have no conflicts of interest to declare.

