
Abstract
Aims: The aim of this study was to analyse the relation between endothelial dysfunction (ED) and the occurrence of radial artery spasm (RAS) during transradial coronary procedures.
Methods and results: From May 2014 to June 2015, endothelial function was assessed by EndoPAT and FMD before the procedure in 165 patients referred for coronary angiography or intervention. The primary endpoint was RAS, defined by patient’s symptoms and procedural characteristics. The mean age of the study population was 63 years and 71% were male. In total 16% of the patients experienced RAS. The incidence of RAS did not differ between patients with and without ED (13.8% vs. 20.2%, OR 0.63, 95% CI: 0.25-1.58, p=0.32). The strongest predictors of RAS were a ratio of radial artery inner diameter and sheath outer diameter smaller than 1 (OR 4.7, 95% CI: 1.35-16.5, p=0.009) and a combination of clinical characteristics presented as an RAS risk score of at least 4 (p=0.007, OR 3.7, 95% CI: 1.37-9.89).
Conclusions: Endothelial dysfunction was not found to be a predictor of the occurrence of radial artery spasm in a cohort of patients undergoing elective heart catheterisation. Radial artery-sheath mismatch is the strongest pre-procedural predictor of RAS.
Abbreviations
BMI: body mass index
CABG: coronary artery bypass graft surgery
CAD: coronary artery disease
DM: diabetes mellitus
ED: endothelial dysfunction
eNOS: endothelial derived nitric oxide synthase
NO: nitric oxide
OR: odds ratio
PAD: peripheral artery disease
PAT: peripheral arterial tonometry
PCI: percutaneous coronary intervention
PORH: post-occlusive reactive hyperaemia
RA: radial artery
RAID/SOD ratio: radial artery internal diameter/sheath outer diameter ratio
RAS: radial artery spasm
RHI: reactive hyperaemia index
SD: standard deviation
TFA: transfemoral approach
TRA: transradial approach
VAS: visual analogue scale
Introduction
Radial artery (RA) access has been used for coronary angiography and interventions since the late 1980s1,2. As a result of superior haemostasis of the superficially located artery, introduction of the transradial approach (TRA) has led to a reduction in access-site bleeding compared to the femoral approach3,4. One of the drawbacks of this technique is the occurrence of radial artery spasm (RAS). This often leads to patient discomfort, procedural failure and catheter friction which may lead to intimal wall injury of the radial artery5-7. The latter can lead to chronic changes of the vessel wall (intimal thickening) and even radial artery occlusion, hampering future procedures8. Some efforts have been made to identify predictors of RAS to prevent this complication9-12; however, the incidence of RAS is still high, varying between 4% and 20%13.
The endothelium has a strong paracrine function and influences smooth muscle cell contraction in the vessel wall. The main characteristic of endothelial dysfunction (ED) is reduced production of nitric oxide (NO) by NO synthase (eNOS) and increased NO breakdown by reactive oxygen species14. This situation can result in paradoxical vasoconstriction to several stimuli. Endothelial dysfunction can be measured non-invasively and predicts future adverse cardiovascular events15,16. Several diagnostic tests are available: most of them are based on increased shear stress after post-occlusive reactive hyperaemia (PORH)17. One of these methods measures reactive hyperaemia in the fingertips by peripheral arterial tonometry (PAT) through the EndoPAT system (Endo-PAT2000; Itamar Medical Ltd., Caesarea, Israel), which has become widely recognised as a reliable method for endothelial function assessment and is a validated predictor of cardiovascular adverse events18,19. Because of an elevated vascular tone, ED might be related to arterial spasm. Indeed, post-TRA transient endothelial dysfunction is detectable20-23, but only one study has described ED before TRA as a predictor of RAS24. In this single study, only one type of endothelial function test was used and the RAS endpoint was defined as a combination of angiographic and clinical features.
We conducted a prospective study to explore the association between ED and a clinical definition of RAS in patients undergoing a transradial coronary procedure.
Methods
STUDY DESIGN AND POPULATION
We conducted this study in a high-volume academic PCI centre. Patients undergoing transradial heart catheterisation were selected for the study. Exclusion criteria were: a) inability to give informed consent; b) acute myocardial infarction; c) haemodynamic instability; d) TR approach within the last 30 days; e) expected transfemoral approach (previous coronary artery bypass grafting, chronic total occlusion, patient preference); f) renal failure with an estimated glomerular filtration rate (eGFR) of <30 ml/min/1.73 m². A total of 796 patients referred for elective intervention were screened from May 2014 to June 2015. One hundred and sixty-five patients were eligible; most patients were not included because of logistic reasons (ambulance transport schedules to referral centres), exclusion criteria and competing studies. After informed consent was obtained, baseline characteristics were noted and pre-procedural flow-mediated dilatation (FMD) of the radial artery and EndoPAT measurements were performed. EndoPAT measurements of 23 patients were not analysable because of noisy signal quality.
The interventionalist was blinded to all study-related tests. The study protocol was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki.
ENDOPAT MEASUREMENTS
The EndoPAT device was used to measure the arterial pulse wave amplitude with pneumatic probes placed on the index finger of each hand, capable of sensing volume changes in the digit with each arterial pulsation. The contralateral hand was used as a reference to correct for potential systemic changes. Volume changes in the fingertip were recorded digitally as pulse amplitude and tracked over time. Brachial occlusion for a period of five minutes was achieved by inflating a blood pressure cuff at the brachium. The reactive hyperaemia index (RHI) was measured: this is the post-to-pre-occlusion PAT signal ratio on the occluded side, divided by the same ratio of the control arm, and further corrected for baseline vascular tone. An RHI with an outcome of <1.68 was considered as endothelial dysfunction25.
FMD MEASUREMENTS
Measurements for achieving FMD were performed with a dedicated ultrasound machine (Sparq; Philips Medical Systems, Andover, MA, USA), using a broadband linear array L12-4 transducer. Ultrasound recordings were made in a cross-sectional view at 5 cm from the styloid process. After baseline measurement of the RA diameter, recordings of the radial artery were made for two to three minutes after occlusion by the pressure cuff, simultaneously with EndoPAT measurements. The maximal diameter at any time following the first few minutes after blood flow restoration was recorded. FMD was expressed as percentage change in radial artery diameter after cuff deflation, compared with the pre-inflation measurement. Measurements were performed by a single operator who was blinded to procedural outcomes.
TRANSRADIAL CORONARY PROCEDURE
All patients received local subcutaneous anaesthesia with 1% lidocaine in the relevant arm. TRA was achieved with a venous cannula technique, and a 6 Fr 10 cm long sheath (Terumo Corp., Tokyo, Japan) was positioned in the radial artery. A standard cocktail of 0.1 mg nitroglycerine and 2.5 mg verapamil was given intra-arterially to reduce spasm. In case of coronary angiography a standard dose of 5,000 IU of unfractionated heparin was given intra-arterially for thrombosis prevention. When a PCI was performed, the amount of unfractionated heparin was adapted to the patient’s body weight. At the end of the procedure, the sheath was removed and a TR band haemostatic device (18 mL TR Band®; Terumo Corp.) was applied, according to the advised protocol of the manufacturer. Procedure characteristics were scored. After each procedure the operator was asked if there was resistance at catheter manipulation or at removal of the catheter or sheath. Patients were asked whether they felt pain in the forearm during the procedure. The amount of self-reported pain was measured on a visual analogue scale (VAS) from 0-10 (“no pain at all” was scored as 0 and “unbearable pain” as 10) at the end of the procedure and before discharge.
After sheath insertion and injection of 200 mg nitroglycerine and 5 mg verapamil the radial artery was used to perform heart catheterisation. To assess the presence of angiographic RAS, angiography was repeated just before sheath removal.
An access-site complication was defined as: vessel dissection and rupture, a haematoma more than 5 cm, pseudoaneurysm, arteriovenous fistula and localised infection. Radial artery occlusion was defined as a reverse Barbeau test type 3 or 4. The procedure was considered successful if the target lesion was treated with or without access-site conversion.
DEFINITIONS OF RADIAL ARTERY SPASM
Clinical RAS was defined as follows: persistent forearm pain (extending beyond the period of catheter manipulation), pain response to catheter manipulation (manoeuvres of the catheter other than withdrawal, such as rotation or small movements to obtain optimal catheter position), pain response to catheter withdrawal, difficult catheter manipulation after being “trapped” by the radial artery and considerable resistance on withdrawal of the sheath. Clinical RAS was present if there were two of the five parameters. Angiographic RAS was defined as follows: the smallest diameter of the radial artery was measured, using the edge detection method by catheter calibration (Philips Xcelera 3.2; Philips Medical Systems, Eindhoven, The Netherlands), and compared to the same segment in the reference angiography. A reduction in diameter of more than 25% compared to the pre-procedural reference diameter was considered as angiographic radial artery spasm26. Combined RAS was defined as RAS with angiographic confirmation.
RADIAL ARTERY SPASM RISK SCORE
An RAS risk score was calculated for all patients. This score was recently published. It consists of five weighted risk factors for radial artery spasm: BMI less than 25 kg/m² (1 point), height less than 170 cm (1 point), current smoking (2 points), hypertension (2 points), PAD (3 points). A predefined score of 4 or more is related to a high risk of RAS10.
RADIAL INTERNAL DIAMETER-SHEATH OUTER DIAMETER RATIO (RAID/SOD RATIO)
The radial artery internal diameter/sheath outer diameter ratio (RAID/SOD ratio) was considered abnormal when smaller than 1.027. The baseline FMD radial artery diameter measurement was used as RAID, and SODs for 5, 6 and 7 Fr sheaths (Radifocus® Introducer II Transradial Kit; Terumo Europe, Leuven, Belgium) were, respectively, 2.29, 2.62 and 2.95 mm.
ENDPOINTS
The difference in clinical RAS occurrence between patients with and without endothelial dysfunction (measured with EndoPAT and FMD) was considered as the primary endpoint. Secondary endpoints were the difference in occurrence of combined RAS in patients with and without endothelial dysfunction (measured with EndoPAT and FMD).
STATISTICAL ANALYSIS
Baseline characteristics are tabulated separately for groups with and without clinical RAS. Continuous variables are presented as mean±standard deviation (SD) in case of a normal distribution and median (interquartile range [IQR]) otherwise. Categorical variables are expressed as frequencies (percentages). Continuous baseline characteristics were compared between groups using an independent samples t-test for normally distributed random variables and the Mann-Whitney test for variables that were not normally distributed. Normality was assessed using histograms and using estimates for skewness and kurtosis. Categorical variables were compared between groups using the chi-square test. All statistical tests were two-tailed, and a p-value of <0.05 was considered statistically significant. A multivariable prediction model for occurrence of RAS was built using stepwise logistic regression with forward selection. Candidate predictors were defined as having a p-value <0.20 in univariate logistic regression analysis. To adhere to the rule of thumb of 10 events per variable, we restricted the number of predictors to be included in the model to a maximum of three. The predictive value of RHI and FMD (as continuous variables) was tested using a ROC analysis. RHI was also tested in a chi-square test using the validated cut-off of 1.68. All statistical analyses were performed with SPSS for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
The mean age of the study population was 63±11 years and 71% were male. Sixteen percent of the patients experienced clinical radial artery spasm, and 27% had angiographic spasm. Clinical spasm confirmed by angiography (combined spasm) occurred in 5% of the patients. Clinical characteristics stratified into clinical RAS or no clinical RAS are presented in Table 1. There were no differences in cardiovascular history, cardiovascular risk factors or medication between the two groups except for patient height.
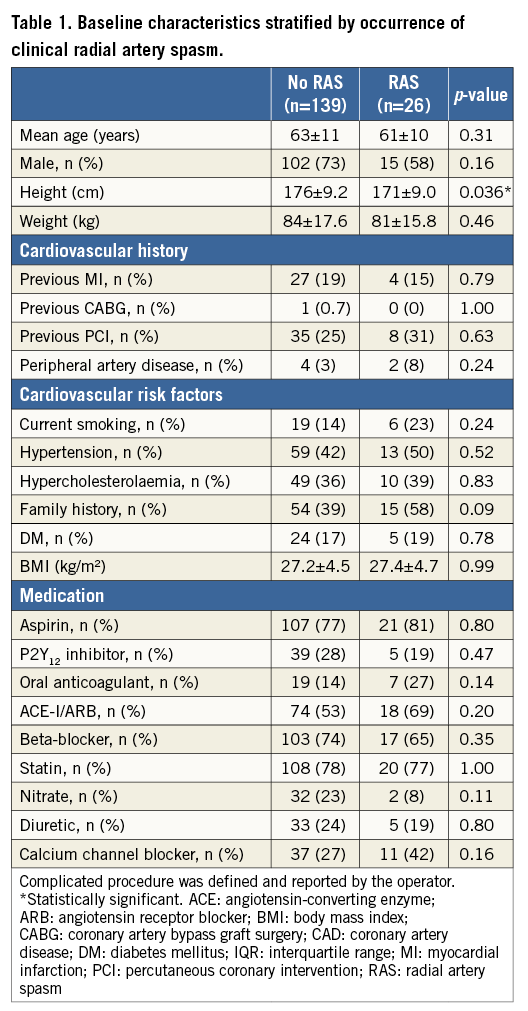
PROCEDURAL CHARACTERISTICS
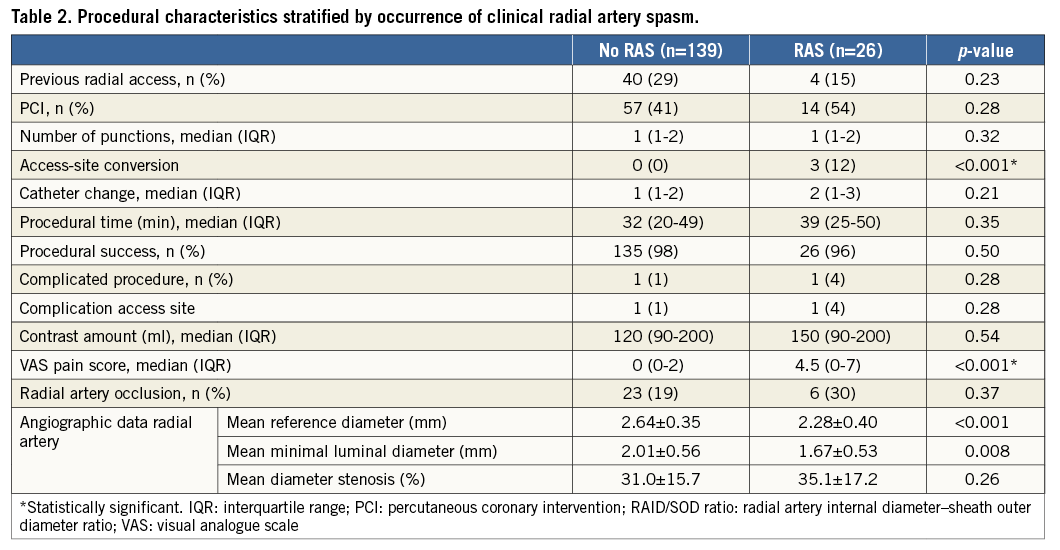
The procedure was successful in 98% of the cases. In three cases the access site had to be converted to the femoral artery; all conversions were related to radial artery spasm. Before catheter advancement, 99% of the patients received a radial artery cocktail and at least 5,000 IU heparin. Two patients had an access-site complication and two other patients experienced a procedural complication. Clinical RAS was not related to previous radial artery access, number of punctures, procedural time, contrast use or number of catheter changes. Patients in the clinical RAS group experienced significantly more pain during the procedure using the VAS pain scale (Table 2).
MEASUREMENTS OF ENDOTHELIAL FUNCTION
The median RHI was 2.01 (IQR 1.48-2.28) in patients with clinical RAS and 1.75 (IQR 1.44-2.34) in those without clinical RAS (p=0.56). In univariate logistic regression analysis, RHI was not found to be associated with the presence of clinical RAS (OR 0.82, 95% CI: 0.71-2.10, p=0.46). The association was still not significant when the validated cut-off of RHI of 1.68 was used to define the presence of ED (OR 0.63, 95% CI: 0.25-1.58, p=0.32) (Figure 1). The mean FMD in patients with clinical RAS was 12.9±13.8% compared to 13.8±11.2% in patients without clinical RAS (p=0.72). Using a previously proposed cut-off value for FMD of 2.95% found in a comparable study24, no difference in clinical RAS was detected either (OR 1.88, 95% CI: 0.67-5.29, p=0.24). Also, a ROC analysis of FMD and continuous RHI values showed poor predictive performance of both tests for the occurrence of RAS (AUC=0.47 and AUC=0.55, respectively).
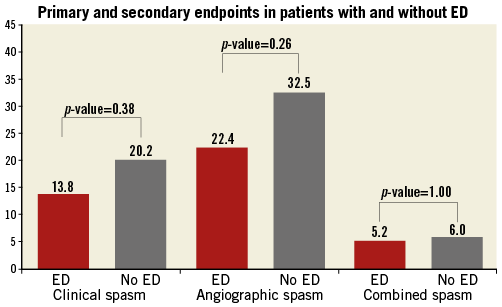
Figure 1. Primary and secondary endpoints in patients with and without ED. The Y-axis represents the percentage of patients with radial artery spasm (RAS). The three different definitions of radial artery spasm are represented on the X-axis using three sets of bars. The red bar represents patients with endothelial dysfunction (ED); the grey bar represents patients without ED. ED is defined as an RHI value <1.68.
ANATOMICAL MEASUREMENTS
There was a strong relation between mean RAID/SOD ratio and clinical RAS (no clinical RAS 0.96±0.20 compared to 0.84±0.17 with clinical RAS, p-value 0.003), and RAID/SOD ratio was a strong predictor when dichotomising the values using a validated cut-off <1.0 (OR 4.7, 95% CI: 1.3-16.5, p=0.01)27.
RAS RISK SCORE
The mean RAS risk score was significantly higher in the clinical spasm group (2.5±1.8 versus 1.8±1.4 in the group without clinical RAS, p=0.03). When using the predefined cut-off (four or more points), there was a good clinical RAS prediction (OR 3.6, 95% CI: 1.4-9.9, p=0.01).
MULTIVARIATE ANALYSIS
Candidate predictors were determined by univariate analysis with p-values <0.2 (female gender, height, family history of CAD, RAS risk score and RAID/SOD ratio). In a forward selection of these candidate predictors for logistic multivariate analysis, only RAID/SOD ratio and the RAS risk score were found to be significant predictors of spasm. When analysed in a multivariate binary regression model, the RAID/SOD ratio <1 was found be the strongest predictor of RAS (OR 5.6, 95% CI: 1.5-21.2, p-value 0.01), followed by the RAS risk score (OR 3.0, 95% CI: 1.0-8.9, p-value 0.051) (Figure 2).
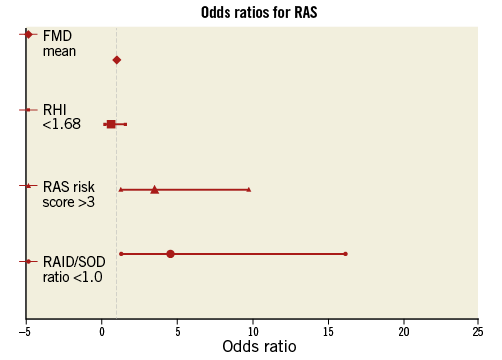
Figure 2. Odds ratios for RAS. The X-axis resembles the odds ratios and 95% confidence intervals. FMD: flow-mediated dilation; RAID/SOD: radial internal diameter/sheath outer diameter; RAS: radial artery spasm; RHI: reactive hyperaemia index
SECONDARY ENDPOINTS
The secondary endpoints are shown in Figure 1. Endothelial dysfunction could not predict angiographic RAS or angiographic RAS combined with clinical RAS. We tested RHI, FMD, RAID/SOD ratio and RAS risk score for prediction of these two alternative definitions of RAS in a univariate and a multivariate analysis, but none of the candidate risk factors was found to be significantly associated with these outcomes. Also, the baseline and procedural variables (Table 1, Table 2) were not significantly different except for gender (more female patients with combined spasm).
Discussion
To our knowledge, this is the first study evaluating the relation between RAS and endothelial dysfunction, measured by EndoPAT. Endothelial dysfunction was not associated with the occurrence of RAS and could not serve as a pre-procedural predictor. A RAID/SOD ratio <1 was the strongest predictor of RAS, followed by the RAS risk score.
Recently, one study evaluating patients with proven coronary artery disease reported a significant difference in pre-procedural FMD when RAS occurred24, but we could not confirm this in our cohort, even when using different outcome definitions of radial artery spasm. Compared to this study, our population also consisted of patients without coronary artery disease and the percentage of diabetic patients was considerably lower. So, the high mean flow-mediated dilatation may reflect less endothelial dysfunction in our cohort and this may have influenced the outcome. Furthermore, our vasodilator cocktail consisted of nitroglycerine that was not given in the aforementioned study. Nevertheless, 40% of our patients had ED according to the validated EndoPAT cut-off value.
There are reports about varying correlations between FMD and RHI measurements in several populations28-30, and there is an indication that EndoPAT and FMD are indicators of different vascular phenomena31, but both tests seem to be indicative of cardiovascular outcome33,34. Nevertheless, in our heterogeneous population, pre-procedural endothelial function measured by either one of the two tests did not predict radial artery spasm.
The incidence of RAS reported in studies is highly variable and strongly influenced by the definition of RAS. We opted for clinical RAS as the primary endpoint, which is patient- and operator-dependent. Though this is a subjective endpoint, patient discomfort and procedural failure are of most clinical importance when RAS occurs. Although evaluating RAS by angiography may appear to be a more objective measurement, angiographic spasm is a very common finding (up to 75% of the cases), and there is a weak relation between angiographic spasm and procedural outcome23. One of the reasons could be the occurrence of diffuse spasm. Although we calculated the maximal diameter stenosis from the minimal luminal post-procedural radial artery diameter and the pre-procedural mean radial artery diameter, pre-procedural radial artery spasm (after sheath insertion) could have influenced our findings.
In our population, RAID/SOD mismatch and RAS risk score were the strongest predictors of clinical RAS. The correlation between known predictors of spasm (gender, height, weight) and radial artery diameter may indicate that mismatch of sheath size is a more important factor in the occurrence of RAS than subtle changes in smooth muscle cell function caused by endothelial dysfunction. Mechanical injury of the vessel inner wall through sheath insertion and catheter friction due to radial artery-sheath mismatch is very common in transradial coronary procedures34. The small impact of endothelial dysfunction compared to mechanical factors to predict RAS is reflected by the odds ratios of the different predictors (Figure 2). Operator experience with TRA and volume load will minimise catheter manipulation and are known to prevent TRA failure due to RAS35. Downsizing coronary catheters and sheaths may further reduce radial artery spasm36. If large bore catheters are mandatory for a procedure, pre-procedural risk assessment by the RAS risk score and determination of sheath mismatch by ultrasound may identify patients at risk and help to choose the most favourable access approach. Such a strategy to prevent TRA procedural failure and patient discomfort has to be validated in future studies.
Conclusion
Endothelial dysfunction was not associated with the occurrence of RAS and could not serve as a pre-procedural predictor. However, RAS could be predicted by the validated RAS risk score and by radial artery/sheath mismatch.
| Impact on daily practice Endothelial dysfunction cannot be used as a predictor of RAS; however, other parameters can predict RAS (RAID/SOD ratio and RAS risk score). |
Acknowledgements
The ultrasound examinations were supported by the Department of Pulmonology, VUmc, Amsterdam, The Netherlands.
Conflict of interest statement
The authors have no conflicts of interest to declare.

